Single-cell dissection of the human brain vasculature
- PMID: 35158371
- PMCID: PMC9680899
- DOI: 10.1038/s41586-022-04521-7
Single-cell dissection of the human brain vasculature
Abstract
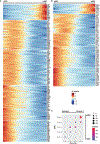
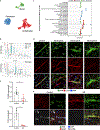
Despite the importance of the cerebrovasculature in maintaining normal brain physiology and in understanding neurodegeneration and drug delivery to the central nervous system1, human cerebrovascular cells remain poorly characterized owing to their sparsity and dispersion. Here we perform single-cell characterization of the human cerebrovasculature using both ex vivo fresh tissue experimental enrichment and post mortem in silico sorting of human cortical tissue samples. We capture 16,681 cerebrovascular nuclei across 11 subtypes, including endothelial cells, mural cells and three distinct subtypes of perivascular fibroblast along the vasculature. We uncover human-specific expression patterns along the arteriovenous axis and determine previously uncharacterized cell-type-specific markers. We use these human-specific signatures to study changes in 3,945 cerebrovascular cells from patients with Huntington's disease, which reveal activation of innate immune signalling in vascular and glial cell types and a concomitant reduction in the levels of proteins critical for maintenance of blood-brain barrier integrity. Finally, our study provides a comprehensive molecular atlas of the human cerebrovasculature to guide future biological and therapeutic studies.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Figures
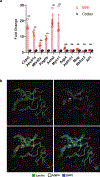


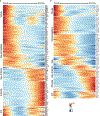
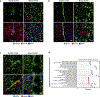
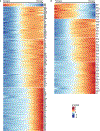








References
MeSH terms
Substances
Grants and funding
- P30 AG072975/AG/NIA NIH HHS/United States
- RF1 AG054012/AG/NIA NIH HHS/United States
- U01 AG61356/NH/NIH HHS/United States
- RF1 AG062377/AG/NIA NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- R01 AG17917/NH/NIH HHS/United States
- R01 AG062335/AG/NIA NIH HHS/United States
- U01 MH119509/MH/NIMH NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- R01 AG15819/NH/NIH HHS/United States
- R01 AG058002/AG/NIA NIH HHS/United States
- R01 HG008155/HG/NHGRI NIH HHS/United States
- P50 HD105351/HD/NICHD NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- UG3 NS115064/NS/NINDS NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- UH3 NS115064/NS/NINDS NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- P30 AG10161/NH/NIH HHS/United States
- U01 NS110453/NS/NINDS NIH HHS/United States
- R56 AG067151/AG/NIA NIH HHS/United States
- R01 AG067151/AG/NIA NIH HHS/United States
- R01 MH109978/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

