B-cell depleters attenuate the humoral response to SARS-CoV-2 vaccines in multiple sclerosis patients: A case-control study
- PMID: 35158480
- PMCID: PMC8599138
- DOI: 10.1016/j.msard.2021.103413
B-cell depleters attenuate the humoral response to SARS-CoV-2 vaccines in multiple sclerosis patients: A case-control study
Abstract
Background: B-cell depleting agents are FDA approved for the treatment of RRMS (ocrelizumab (OCR) and ofatumumab (OFA)) and PPMS (OCR). In the case of OCR, prior studies have raised concerns about patients' ability to form antibodies in response to various antigens, especially SARS-CoV-2. In addition, emerging data have shown an attenuated humoral response to vaccines against SARS-CoV-2. The objective of this study is to determine whether b-cell depleters or sphingosine 1-phosphate (S1P) modulators attenuate the antibody response to various SARS-CoV-2 vaccines in patients with MS as compared with other MS disease modifying therapies (DMTs).
Methods: This is a case-control study looking at the odds of developing antibodies to three SARS-CoV-2 vaccines (Pfizer-BioNTech, Moderna, and Johnson & Johnson) in patients treated with b-cell depleters or S1P modulators versus other disease modifying therapies. Patients were recruited at the Comprehensive MS Center at Methodist Hospitals. Patients who did not have a prior COVID-19 infection and received one of the three vaccines were tested for antibodies against the SARS-CoV-2 spike protein (Labcorp, semi-quantitative total antibody) at least two weeks following the final dose of the vaccine. Groups (B-cell, S1P modulators, other DMT, and no DMT) were compared on antibody level. The main outcome was whether or not a humoral response was detected by antibody testing. Dichotomous antibody response was tested using logistic regression models, and the quantitative response was tested using ANCOVA adjusted for covariates (age, sex, race, MS type, disease duration, vaccine, and lymphocyte count). P-values <0.05 were considered significant.
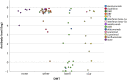
Results: Sixty-seven patients were enrolled in the study, with 17 on OCR, 3 on OFA, 12 on S1P modulators, 29 on other DMT, and 6 not currently on any DMT. Patients who received OCR or OFA had decreased odds of forming antibodies (OR 0.031, p < 0.001, 95% CI (0.003-0.268)). Patients who received S1P modulators did not have decreased odds of forming antibodies (OR 0.413, p = 0.413, 95% CI (0.28-21.7). However, when analyzing the antibody response as a continuous variable, patients on S1P modulators showed lower absolute levels of antibodies (p = 0.024).
Conclusions: Patients who received B-cell depleters within the prior 6 months of SARS-CoV-2 vaccination had decreased odds of developing antibodies compared with other DMTs. In line with other similar research, this suggests that b-cell depleters attenuate the antibody response to SARS-CoV-2 vaccines. Although S1P modulators had an attenuation of the absolute antibody level, the odds of being negative did not differ from those on other DMTs.
Keywords: COVID-19; Disease modifying therapies; Ocrelizumab; SARS-CoV-2; Vaccine.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Research funding from Novartis unrelated to the current study. Consultant fees from Bayer, Biogen, Bristol Meyers Squib, Genentech, Novartis, and Sanofi Genzyme. Speaking fees from AbbVie, Alexion, Biogen, Bristol Meyers Squib, EMD Serono, Genentech, Janssen, Novartis, and Sanofi Genzyme.
Figures
References
-
- Achiron, A., Mandel, M., Dreyer-Alster, S., Harari, G., Magalashvili, D., Sonis, P., Dolev, M., Menascu, S., Flechter, S., Falb, R., Gurevich, M., 2021. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies: 10.1177/17562864211012835 14. 10.1177/17562864211012835. - DOI - DOI - PMC - PubMed
-
- Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., Manfrini M., McNamara J., Robertson D.S., Stokmaier D., Wendt J.K., Winthrop K.L., Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology. 2020;95:e1999–e2008. doi: 10.1212/WNL.0000000000010380. - DOI - PMC - PubMed
-
- Bingham C.O., Looney R.J., Deodhar A., Halsey N., Greenwald M., Codding C., Trzaskoma B., Martin F., Agarwal S., Kelman A. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64–74. doi: 10.1002/art.25034. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous