Porcine Intestinal Apical-Out Organoid Model for Gut Function Study
- PMID: 35158695
- PMCID: PMC8833427
- DOI: 10.3390/ani12030372
Porcine Intestinal Apical-Out Organoid Model for Gut Function Study
Abstract
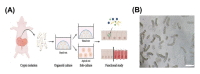
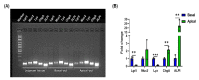
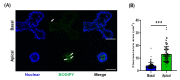
Pig models provide valuable research information on farm animals, veterinary, and biomedical sciences. Experimental pig gut models are used in studies on physiology, nutrition, and diseases. Intestinal organoids are powerful tools for investigating intestinal functions such as nutrient uptake and gut barrier function. However, organoids have a basal-out structure and need to grow in the extracellular matrix, which causes difficulties in research on the intestinal apical membrane. We established porcine intestinal organoids from jejunum tissues and developed basal-out and apical-out organoids using different sub-culture methods. Staining and quantitative real-time PCR showed the difference in axis change of the membrane and gene expression of epithelial cell marker genes. To consider the possibility of using apical-out organoids for intestinal function, studies involving fatty acid uptake and disruption of the epithelial barrier were undertaken. Fluorescence fatty acid was more readily absorbed in apical-out organoids than in basal-out organoids within the same time. To determine whether apical-out organoids form a functional barrier, a fluorescent dextran diffusion assay was performed. Hence, we successfully developed porcine intestinal organoid culture systems and showed that the porcine apical-out organoid model is ideal for the investigation of the intestinal environment. It can be used in future studies related to the intestine across various research fields.
Keywords: apical-out; barrier integrity; intestinal organoid; nutrient uptake; pig model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

