A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY
- PMID: 35158827
- PMCID: PMC8833795
- DOI: 10.3390/cancers14030559
A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY
Abstract
Background: We evaluated the safety and efficacy of direct oral anticoagulants (DOACs) versus subcutaneous dalteparin for cancer-associated venous thromboembolism (CA-VTE) in patients with advanced upper gastrointestinal (GI) tract, hepatobiliary, or pancreatic cancer.
Methods: This was a multicenter, randomized, open-label, phase II trial in five centers. Patients randomly received rivaroxaban (15 mg twice daily for 3 weeks, then 20 mg once daily)/apixaban (10 mg twice daily for the first 7 days, then 5 mg twice daily) or dalteparin (200 IU/kg once daily for the first month, then 150 IU/kg once daily). Randomization was stratified by the Eastern Cooperative Oncology Group Performance Status, primary cancer type, active chemotherapy, and participating centers. The primary endpoint was the rates of clinically relevant bleeding (CRB) in the full analysis set (FAS).
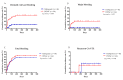
Results: A total of 90 patients were randomly assigned to the DOAC (n = 44) and dalteparin groups (n = 46) in FAS. CRB and major bleeding (MB) rates were 34.1% and 13.0% (p = 0.018) and 18.2% and 4.3% (p = 0.047) for the DOAC and dalteparin groups, respectively. Time to CRB and MB was higher in the DOAC group than in the dalteparin group (hazard ratio [HR] 2.83; p = 0.031 and HR 4.32; p = 0.064). Cancer involvement at the GI mucosa was also a significant risk factor for CRB. Recurrent CA-VTE occurred in 2.3% and 2.2% of patients given DOAC and dalteparin, respectively (p = 1.000).
Conclusion: DOAC therapy further increased the risk of bleeding compared with dalteparin in patients with active advanced upper GI tract, hepatobiliary, or pancreatic cancer, suggesting that extra caution should be taken when selecting anticoagulants for CA-VTE.
Keywords: apixaban; dalteparin; gastrointestinal tract cancer; hepatobiliary cancer; pancreatic cancer; rivaroxaban; venous thromboembolism.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Prandoni P., Lensing A.W., Piccioli A., Bernardi E., Simioni P., Girolami B., Marchiori A., Sabbion P., Prins M.H., Noventa F., et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. - DOI - PubMed
-
- Hutten B.A., Prins M.H., Gent M., Ginsberg J., Tijssen J.G., Büller H.R. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000;18:3078–3083. doi: 10.1200/JCO.2000.18.17.3078. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

