High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC
- PMID: 35158841
- PMCID: PMC8833516
- DOI: 10.3390/cancers14030573
High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC
Abstract
Introduction: Thoracic re-irradiation for recurrent lung cancer dates back four decades, when the first small series on 29 patients receiving palliative doses was published. With 5-year overall survival rates of 57% in PDL-1 positive patients after primary chemo-radio-immunotherapy, the number of patients who experience loco-regional relapse will increase in the near future. In this context, centrally recurring lung tumors pose a major treatment challenge. Hence, the aim of the current review is to compile the available evidence on curatively intended thoracic re-irradiation for this special clinical situation.
Methods: A systematic literature search according to the PRISMA guidelines was performed. A study was included when the following criteria were met: (1) 66% of the patients had NSCLC, (2) a total dose of 50 Gy in the second course and/or a biologically effective dose of at least 100 Gy in both treatment courses was administered, (3) re-irradiation was administered with modern radiation techniques, (4) 50% or more of the patients had a centrally located relapse, (5) the minimum cohort size was 30 patients.
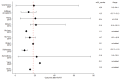
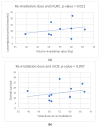
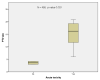
Results: Of the initial 227 studies, 11 were analyzed, 1 of which was prospective. Median overall survival (OS) was 18.1 months (range 9.3-25.1), median progression free survival (PFS) was nine months (range 4.5-16), and median loco-regional control (LRC) was 12.1 months (range 6.5-20). Treatment-related mortality rates ranged from 2% to 14%. The total dose at re-irradiation correlated with both LRC (p-value = 0.012) and OS (p-value = 0.007) with a close relation between these two clinical endpoints (p-value = 0.006). The occurrence of acute toxicity grade 1 to 4 depended on the PTV size at re-irradiation (p-value = 0.033).
Conclusion: The evidence regarding curative re-irradiation for centrally recurrent NSCLC is primarily based on scarce retrospective data, which are characterized by a high degree of heterogeneity. The OS in this clinically challenging situation is expected to be around 1.5 years after re-treatment. Patients with a good performance score, younger age, small tumors, and a longer interval to recurrence potentially benefit most from re-irradiation. In this context, prospective trials are warranted to achieve substantial advances in the field.
Keywords: NSCLC; immunotherapy; lung cancer; re-irradiation; stage III.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Vansteenkiste J., De Ruysscher D., Eberhardt W.E., Lim E., Senan S., Felip E., Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24((Suppl. 6)):vi89–vi98. doi: 10.1093/annonc/mdt241. - DOI - PubMed
-
- Chao H.H., Berman A.T., Simone C.B., 2nd, Ciunci C., Gabriel P., Lin H., Both S., Langer C., Lelionis K., Rengan R., et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017;12:281–292. doi: 10.1016/j.jtho.2016.10.018. - DOI - PubMed
-
- Curran W.J., Paulus R., Langer C.J., Komaki R., Lee J.S., Hauser S., Movsas B., Wasserman T., Rosenthal S.A., Gore E., et al. Sequential vs Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J. Natl. Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

