Ex Vivo Fluorescence Confocal Microscopy in Specimens of the Liver: A Proof-of-Concept Study
- PMID: 35158859
- PMCID: PMC8833349
- DOI: 10.3390/cancers14030590
Ex Vivo Fluorescence Confocal Microscopy in Specimens of the Liver: A Proof-of-Concept Study
Abstract
Ex vivo Fluorescence Confocal Microscopy (FCM) is a technique providing high-resolution images of native tissues. The method is increasingly used in surgical settings in areas of dermatology and urology. Only a few publications exist about examinations of tumors and non-neoplastic lesions of the liver. We report on the application of FCM in biopsies, surgical specimens and autopsy material (33 patients, 39 specimens) of the liver and compare the results to conventional histology. Our preliminary examinations indicated a perfect suitability for tumor diagnosis (ĸ = 1.00) and moderate/good suitability for the assessment of inflammation (ĸ = 0.4-0.6) with regard to their severity and localization. Macro-vesicular steatosis was reliably detected, micro-vesicular steatosis tended to be underestimated. Cholestasis and eosinophilic granules in granulocytes were not represented in the scans. The tissue was preserved as native material and maintained its quality for downstream histological, immunohistological and molecular examinations. In summary, FCM is a material sparing method that provides rapid feedback to the clinician about the presence of tumor, the degree of inflammation and structural changes. This can lead to faster therapeutic decisions in the management of liver tumors, treatment of hepatitis or in liver transplant medicine.
Keywords: digital pathology; fluorescence confocal microscopy; hepatitis; liver biopsies; liver transplantation; liver tumors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





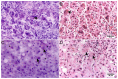

Comment in
-
Fluorescence confocal microscopy on liver specimens: Could it be a game changer in transplantation pathology?Liver Transpl. 2023 Sep 1;29(9):915-916. doi: 10.1097/LVT.0000000000000159. Epub 2023 Apr 17. Liver Transpl. 2023. PMID: 37057813 No abstract available.
References
-
- Wang M., Kimbrell H.Z., Sholl A.B., Tulman D.B., Elfer K.N., Schlichenmeyer T.C., Lee B.R., Lacey M., Brown J.Q. High-Resolution Rapid Diagnostic Imaging of Whole Prostate Biopsies Using Video-Rate Fluorescence Structured Illumination Microscopy. Cancer Res. 2015;75:4032–4041. doi: 10.1158/0008-5472.CAN-14-3806. - DOI - PMC - PubMed
-
- Yadav R., Mukherjee S., Hermen M., Tan G., Maxfield F.R., Webb W.W., Tewari A.K. Multiphoton microscopy of prostate and periprostatic neural tissue: A promising imaging technique for improving nerve-sparing prostatectomy. J. Endourol. 2009;23:861–867. doi: 10.1089/end.2009.0221. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

