Update on Management Recommendations for Advanced Cutaneous Squamous Cell Carcinoma
- PMID: 35158897
- PMCID: PMC8833756
- DOI: 10.3390/cancers14030629
Update on Management Recommendations for Advanced Cutaneous Squamous Cell Carcinoma
Abstract
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer, the incidence of which has risen over the last years. Although cSCC rarely metastasizes, early detection and treatment of primary tumours are critical to limit progression and local invasion. Several prognostic factors related to patients' clinicopathologic profile and tumour features have been identified as high-risk markers and included in the stratification scales, but their association with regional control or survival is uncertain. Therefore, decision-making on the diagnosis and management of cSCC should be made based on each individual patient's characteristics. Recent advances in non-invasive imaging techniques and molecular testing have enhanced clinical diagnostic accuracy. Surgical excision is the mainstay of local treatment, whereas radiotherapy (RT) is recommended for patients with inoperable disease or in specific circumstances. Novel systemic treatments including immunotherapies and targeted therapies have changed the therapeutic landscape for cSCC. The anti-PD-1 agent cemiplimab is currently the only FDA/EMA-approved first-line therapy for patients with locally advanced or metastatic cSCC who are not candidates for curative surgery or RT. Given the likelihood of recurrence and the increased risk of developing multiple cSCC, close follow-up should be performed during the first years of treatment and continued long-term surveillance is warranted.
Keywords: cutaneous squamous cell carcinoma; multidisciplinary management; prognosis; surgery; systemic therapy.
Conflict of interest statement
J. Garcia-Foncillas discloses consulting/advisory/honoraria speaker roles with Abbott, Amgen, Astellas, Astra Zeneca, Biocartis, Boehringer Ingelheim, BMS, Bayer, Celgene, Eisai, Foundation Medicine, GSK, Hospira, Janssen, Lilly, Merck Serono, MSD, Novartis, Pharmamar, Pfizer, Roche, Sanofi, Servier, Sysmex, and Tesaro. R. Mesía discloses consulting/advisory/honoraria speaker role with Amgen, BMS, Merck Serono, MSD, Roche, and Sanofi. O. Sanmartín discloses consulting/advisory/honoraria speaker role with Roche, ISDIN, and Sanofi. F. Rojo has received honorary for consultancy from Roche, BMS, MSD, Merck, Novartis, Pfizer, Astra Zeneca, Abbvie, Pierre Fabre, InCyte, and Bayer. J. Mestre discloses consulting/advisory/honoraria speaker roles with Lavigor, Roche, and Sanofi. A. Tejera has received honoraria for consultancy from Sanofi. S. Martín discloses consulting/advisory/honoraria speaker roles with MSD, Sanofi/Regeneron, BMS, Novartis, Roche, and Pierre-Fabre. I. Azinovic discloses no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

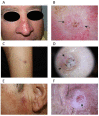
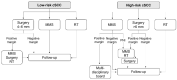
References
-
- Tejera-Vaquerizo A., Descalzo-Gallego M.A., Otero-Rivas M.M., Posada-García C., Rodríguez-Pazos L., Pastushenko I., Marcos-Gragera R., García-Doval I. Skin Cancer Incidence and Mortality in Spain: A Systematic Review and Meta-Analysis. Actas Dermosifiliogr. 2016;107:318–328. doi: 10.1016/j.ad.2015.12.008. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

