Incident Colorectal Cancer in Inflammatory Bowel Disease
- PMID: 35158989
- PMCID: PMC8833396
- DOI: 10.3390/cancers14030721
Incident Colorectal Cancer in Inflammatory Bowel Disease
Abstract
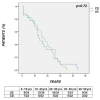
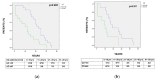
Colorectal cancer (CRC) risk is increased in Inflammatory Bowel Disease (IBD) and surveillance needs to be tailored according to individual risk. The open issues include the role of the characteristics of IBD and CRC in determining the long-term outcome. These issues were assessed in our multicenter study, including a cohort of 56 IBD patients with incident CRC. The clinical and histopathological features of IBD patients and of CRC were recorded. Incident CRC in IBD occurred at a young age (≤40 years) in 25% of patients (median age 55.5 (22-76)). Mucinous signet-ring carcinoma was detected in 6 out of the 56 (10.7%) patients, including 4 with Ulcerative Colitis (UC) and 2 with Crohn's disease (CD). CRC was more frequently diagnosed by colonoscopy in UC (85.4% vs. 50%; p = 0.01) and by imaging in Crohn's Disease CD (5.8% vs. 31.8%; p = 0.02). At onset, CRC-related symptoms occurred in 29 (51.9%) IBD patients. The time interval from the diagnosis of IBD to CRC was shorter in UC and CD patients with >40 years (p = 0.002; p = 0.01). CRC-related death occurred in 10 (29.4%) UC and in 6 (27.2%) CD patients (p = 0.89), with a short time interval from CRC to death (UC vs. CD: 6.5 (1-68) vs. 14.5 (8-40); p = 0.85; IBD: 12 months (1-68)). CRC occurring at a young age, a short time interval from the diagnosis of IBD to CRC-related death in the elderly, CRC-symptoms often mimicking IBD relapse and the observed high mortality rate may support the need of closer surveillance intervals in subgroups of patients.
Keywords: Inflammatory Bowel Disease; clinical outcome; colorectal cancer; incident cancer.
Conflict of interest statement
The study was not supported by any grant and any of the below reported disclosures are related to the study. L.B.: Lecture fees and/or Advisory Board for Janssen, AbbVie, Ferring, Pfizer, Takeda. A.A. received consultancy fees from AbbVie, Allergan, Amgen, Arena, Biogen, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, M.S.D., Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda; lecture fees from AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Ferring, Galapagos, Gilead, Janssen, M.S.D., Mitsubishi Tanabe, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, Tigenix; research grants from M.S.D., Pfizer, Takeda; M.L.S.: lecture fees and/or advisory board for Celltrion, Janssen, Pfizer, Takeda; F.C.: Lecture fees/Adv. Board: Takeda, Pfizer, Janssen, Biogen, Fresenius, Sandoz; F.M. Grant: Pfizer, AbbVie, Jansen. A.O. received lecture grants and/or served as an advisory board member for: AbbVie, Chiesi, Janssen, Galapagos, M.S.D., Pfizer, Samsung Bioepis, Sofar and Takeda Pharmaceuticals. P.A.: Conference fee from Bioprojet Nutricia; G.R.: Advisory Board: M.S.D., Janssen, AbbVie, Takeda; W.F.: Advisory boards or fundings from Ferring Italia, Janssen, Sandoz, Pfizer, Takeda, Abbvie; F.M.: Lecture fees: Pfizer, Takeda e Jannsen. L.G.: Consulting and/or lecture fees: AbbVie, Janssen, M.S.D., Shire, Takeda, Pfizer, Vifor Pharma. A.T.: Lecture fees: Takeda and Jannsen. S.R.: Lecture fees from AbbVie, M.S.D., Takeda Pharmaceuticals, Janssen and Pfizer. The remaining authors disclose no conflicts of interest.
Figures



References
-
- Brentnall T., Haggitt R., Rabinovitch P., Kimmey M., Bronner M., Levine D., Kowdley K., Stevens A., Crispin D., Emond M., et al. Risk and natural history of colonic neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1996;110:331–338. doi: 10.1053/gast.1996.v110.pm8566577. - DOI - PubMed
LinkOut - more resources
Full Text Sources

