Somatostatin Receptor 2 Expression Profiles and Their Correlation with the Efficacy of Somatostatin Analogues in Gastrointestinal Neuroendocrine Tumors
- PMID: 35159042
- PMCID: PMC8834049
- DOI: 10.3390/cancers14030775
Somatostatin Receptor 2 Expression Profiles and Their Correlation with the Efficacy of Somatostatin Analogues in Gastrointestinal Neuroendocrine Tumors
Abstract
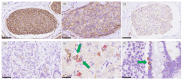
Somatostatin analogues (SSAs) are widely used to treat gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Somatostatin receptor 2 (SSTR2) immunoreactivity serves as a predictive marker of the therapeutic efficacy of SSAs in pancreatic NETs. However, SSTR2 expression profiles in tumor cells and their association with the therapeutic efficacy of SSAs remains virtually unknown in gastrointestinal NETs (GI-NETs). Therefore, we evaluated the association between SSTR2 immunoreactivity and embryological origin and proliferative activity in 132 resected surgical tissues of GI-NETs. The correlation between SSAs' therapeutic efficacy and SSTR2 immunoreactivity was evaluated in 14 GI-NETs treated with SSAs. SSTR2 immunoreactivity was evaluated using Volante scores, immunoreactive scores, and digital image analysis (DIA). SSTR2 immunoreactivity was significantly negatively and positively correlated with the Ki-67 labeling index in foregut and hindgut NETs, respectively. In the normal mucosa, neuroendocrine cells in the rectum had significantly lower positive rates of SSTR2 than those in the stomach and duodenum. SSTR2 expression profiles in GI-NETs could differ by primary sites, while the difference of those between foregut and hindgut NETs might be derived from the SSTR2 status of normal neuroendocrine cell counterparts. In addition, DIA could provide a good alternative for predicting response to SSAs in evaluating SSTR2 immunoreactivity of GI-NETs.
Keywords: digital image analysis; foregut NET; hindgut NET; immunohistochemistry; neuroendocrine tumor; somatostatin receptor 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Watanabe H., Yamazaki Y., Fujishima F., Izumi K., Imamura M., Hijioka S., Toriyama K., Yatabe Y., Kudo A., Motoi F., et al. O(6)-methylguanine DNA methyltransferase and glucose transporter 2 in foregut and hindgut gastrointestinal neuroendocrine neoplasms. BMC Cancer. 2020;20:1195. doi: 10.1186/s12885-020-07579-6. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

