Targeting Mitochondrial COX-2 Enhances Chemosensitivity via Drp1-Dependent Remodeling of Mitochondrial Dynamics in Hepatocellular Carcinoma
- PMID: 35159089
- PMCID: PMC8834292
- DOI: 10.3390/cancers14030821
Targeting Mitochondrial COX-2 Enhances Chemosensitivity via Drp1-Dependent Remodeling of Mitochondrial Dynamics in Hepatocellular Carcinoma
Abstract
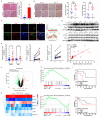
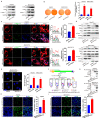
Mitochondria are highly dynamic organelles and undergo constant fission and fusion, which are both essential for the maintenance of cell physiological functions. Dysregulation of dynamin-related protein 1 (Drp1)-dependent mitochondrial dynamics is associated with tumorigenesis and the chemotherapeutic response in hepatocellular carcinoma (HCC). The enzyme cyclooxygenase-2 (COX-2) is overexpressed in most cancer types and correlates with a poor prognosis. However, the roles played by the translocation of mitochondrial COX-2 (mito-COX-2) and the interaction between mito-COX-2 and Drp1 in chemotherapeutic responses remain to be elucidated in the context of HCC. Bioinformatics analysis, paired HCC patient specimens, xenograft nude mice, immunofluorescence, transmission electron microscopy, molecular docking, CRISPR/Cas9 gene editing, proximity ligation assay, cytoplasmic and mitochondrial fractions, mitochondrial immunoprecipitation assay, and flow cytometry analysis were performed to evaluate the underlying mechanism of how mito-COX-2 and p-Drp1Ser616 interaction regulates the chemotherapeutic response via mitochondrial dynamics in vitro and in vivo. We found that COX-2 and Drp1 were frequently upregulated and confer a poor prognosis in HCC. We also found that the proportion of mito-COX-2 and p-Drp1Ser616 was increased in HCC cell lines. In vitro, we demonstrated that the enhanced mitochondrial translocation of COX-2 promotes its interaction with p-Drp1Ser616 via PTEN-induced putative kinase 1 (PINK1)-mediated Drp1 phosphorylation activation. This increase was associated with higher colony formation, cell proliferation, and mitochondrial fission. These findings were confirmed by knocking down COX-2 in HCC cells using CRISPR/Cas9 technology. Furthermore, inhibition of Drp1 using pharmacologic inhibitors (Mdivi-1) or RNA interference (siDNM1L) decreased mito-COX-2/p-Drp1Ser616 interaction-mediated mitochondrial fission, and increased apoptosis in HCC cells treated with platinum drugs. Moreover, inhibiting mito-COX-2 acetylation with the natural phytochemical resveratrol resulted in reducing cell proliferation and mitochondrial fission, occurring through upregulation of mitochondrial deacetylase sirtuin 3 (SIRT3), which, in turn, increased the chemosensitivity of HCC to platinum drugs in vitro and in vivo. Our results suggest that targeting interventions to PINK1-mediated mito-COX-2/p-Drp1Ser616-dependent mitochondrial dynamics increases the chemosensitivity of HCC and might help us to understand how to use the SIRT3-modulated mito-COX-2/p-Drp1Ser616 signaling axis to develop an effective clinical intervention in hepatocarcinogenesis.
Keywords: apoptosis; dynamin-related protein 1; hepatocellular carcinoma; mitochondrial cyclooxygenase-2; mitochondrial dynamics; sirtuin 3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Chu Y.J., Yang H.I., Wu H.C., Lee M.H., Liu J., Wang L.Y., Lu S.N., Jen C.L., You S.L., Santella R.M., et al. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. Eur. J. Cancer. 2018;94:37–46. doi: 10.1016/j.ejca.2018.02.010. - DOI - PMC - PubMed
-
- Chen J.H., Wang Y.Y., Lv W.B., Gan Y., Chang W., Tian N.N., Huang X.H., Liu L., Yu X.F., Chen S.D. Effects of interactions between environmental factors and KIF1B genetic variants on the risk of hepatocellular carcinoma in a Chinese cohort. World J. Gastroenterol. 2016;22:4183–4190. doi: 10.3748/wjg.v22.i16.4183. - DOI - PMC - PubMed
Grants and funding
- 81874272, 81973082, 82073588, and 81773465/National Natural Science Foundation of China
- 2017YFA0205201/Major State Basic Research Development Program of China
- 2017KF09/Open Research Fund of State Key Laboratory of Molecular Vaccinology and Mo-lecular Diagnostics
- 201810384011/the XMU Training Program of Innovation and Enterpreneurship for Undergraduates
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

