Enhanced Loss of Retinoic Acid Network Genes in Xenopus laevis Achieves a Tighter Signal Regulation
- PMID: 35159137
- PMCID: PMC8834563
- DOI: 10.3390/cells11030327
Enhanced Loss of Retinoic Acid Network Genes in Xenopus laevis Achieves a Tighter Signal Regulation
Abstract
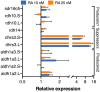
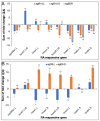
Retinoic acid (RA) is a major regulatory signal during embryogenesis produced from vitamin A (retinol) by an extensive, autoregulating metabolic and signaling network to prevent fluctuations that result in developmental malformations. Xenopus laevis is an allotetraploid hybrid frog species whose genome includes L (long) and S (short) chromosomes from the originating species. Evolutionarily, the X. laevis subgenomes have been losing either L or S homoeologs in about 43% of genes to generate singletons. In the RA network, out of the 47 genes, about 47% have lost one of the homoeologs, like the genome average. Interestingly, RA metabolism genes from storage (retinyl esters) to retinaldehyde production exhibit enhanced gene loss with 75% singletons out of 28 genes. The effect of this gene loss on RA signaling autoregulation was studied. Employing transient RA manipulations, homoeolog gene pairs were identified in which one homoeolog exhibits enhanced responses or looser regulation than the other, while in other pairs both homoeologs exhibit similar RA responses. CRISPR/Cas9 targeting of individual homoeologs to reduce their activity supports the hypothesis where the RA metabolic network gene loss results in tighter network regulation and more efficient RA robustness responses to overcome complex regulation conditions.
Keywords: Xenopus; gene duplication; gene regulation; genome evolution; homoeolog; retinoic acid; signaling robustness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

