Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients
- PMID: 35159144
- PMCID: PMC8834389
- DOI: 10.3390/cells11030334
Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients
Abstract
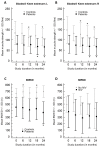
Late-onset Pompe disease (LOPD) is a rare, progressive disorder characterized by limb-girdle muscle weakness and/or respiratory insufficiency, caused by acid alpha-glucosidase (GAA) gene mutations and treated with enzyme replacement therapy. We studied isometric muscle strength in eight muscle groups bilaterally using a Biodex® dynamometer, as well as the Medical Research Council sum score (MRC-SS), hand grip strength, 6 min walk distance (6MWD), 10 m walk test (10MWT) and timed up-and-go test (TUG) in 12 adult, ambulatory, treated LOPD patients and 12 age-/gender-matched healthy controls, every 6 months for 2 years. The mean isometric muscle strength showed a significant decline in right and left knee extensors at 12 months in controls (p < 0.014; p < 0.016), at 18 months in patients (p < 0.010; p < 0.007) and controls (only right side, p < 0.030) and at 24 months in both groups (p < 0.035). The mean 6MWD in patients significantly decreased after 24 months, from 451.9 m to 368.1 m (p < 0.003), whereas in controls, the mean 6MWD significantly increased after 6 months (p < 0.045) and 18 months (p < 0.020) (at 24 months p = 0.054). In patients and controls, the MRC-SS, hand grip test, 10MWT and TUG did not show significant changes (p > 0.05). We conclude that the 6MWD is a useful outcome measure to detect motor decline in treated LOPD patients.
Keywords: 6MWD; Biodex® dynamometer; ERT; GSD2; LOPD; enzyme replacement therapy; glycogen storage disease type 2; isometric; longitudinal; muscle strength.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Van der Ploeg A.T., Kruijshaar M.E., Toscano A., Laforêt P., Angelini C., Lachmann R.H., Pascual S.I., Roberts M., Rösler K., Stulnig T., et al. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: A 10-year experience. Eur. J. Neurol. 2017;24:768.e31. doi: 10.1111/ene.13285. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

