Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle
- PMID: 35159148
- PMCID: PMC8834403
- DOI: 10.3390/cells11030338
Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle
Abstract
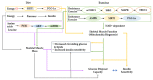
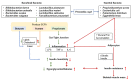
Skeletal muscle mass plays a critical role in a healthy lifespan by helping to regulate glucose homeostasis. As seen in sarcopenia, decreased skeletal muscle mass impairs glucose homeostasis, but it may also be caused by glucose dysregulation. Gut microbiota modulates lipopolysaccharide (LPS) production, short-chain fatty acids (SCFA), and various metabolites that affect the host metabolism, including skeletal muscle tissues, and may have a role in the sarcopenia etiology. Here, we aimed to review the relationship between skeletal muscle mass, glucose homeostasis, and gut microbiota, and the effect of consuming probiotics and prebiotics on the development and pathological consequences of sarcopenia in the aging human population. This review includes discussions about the effects of glucose metabolism and gut microbiota on skeletal muscle mass and sarcopenia and the interaction of dietary intake, physical activity, and gut microbiome to influence sarcopenia through modulating the gut-muscle axis. Emerging evidence suggests that the microbiome can regulate both skeletal muscle mass and function, in part through modulating the metabolisms of short-chain fatty acids and branch-chain amino acids that might act directly on muscle in humans or indirectly through the brain and liver. Dietary factors such as fats, proteins, and indigestible carbohydrates and lifestyle interventions such as exercise, smoking, and alcohol intake can both help and hinder the putative gut-muscle axis. The evidence presented in this review suggests that loss of muscle mass and function are not an inevitable consequence of the aging process, and that dietary and lifestyle interventions may prevent or delay sarcopenia.
Keywords: glucose metabolism; gut microbiome; gut microbiota-muscle axis; inflammation; short-chain fatty acids; skeletal muscle mass.
Conflict of interest statement
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Lampignano L., Bortone I., Castellana F., Donghia R., Guerra V., Zupo R., De Pergola G., Di Masi M., Giannelli G., Lozupone M., et al. Impact of Different Operational Definitions of Sarcopenia on Prevalence in a Population-Based Sample: The Salus in Apulia Study. Int. J. Environ. Res. Public Health. 2021;18:12979. doi: 10.3390/ijerph182412979. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

