Essential Roles of Efferent Duct Multicilia in Male Fertility
- PMID: 35159149
- PMCID: PMC8834061
- DOI: 10.3390/cells11030341
Essential Roles of Efferent Duct Multicilia in Male Fertility
Abstract
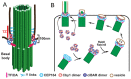
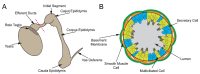
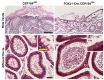
Cilia are microtubule-based hair-like organelles on the cell surface. Cilia have been implicated in various biological processes ranging from mechanosensation to fluid movement. Ciliary dysfunction leads to a plethora of human diseases, known as ciliopathies. Although non-motile primary cilia are ubiquitous, motile multicilia are found in restricted locations of the body, such as the respiratory tract, the oviduct, the efferent duct, and the brain ventricles. Multicilia beat in a whip-like motion to generate fluid flow over the apical surface of an epithelium. The concerted ciliary motion provides the driving force critical for clearing airway mucus and debris, transporting ova from the ovary to the uterus, maintaining sperm in suspension, and circulating cerebrospinal fluid in the brain. In the male reproductive tract, multiciliated cells (MCCs) were first described in the mid-1800s, but their importance in male fertility remained elusive until recently. MCCs exist in the efferent ducts, which are small, highly convoluted tubules that connect the testis to the epididymis and play an essential role in male fertility. In this review, we will introduce multiciliogenesis, discuss mouse models of male infertility with defective multicilia, and summarize our current knowledge on the biological function of multicilia in the male reproductive tract.
Keywords: cilia; efferent ducts; fertility; multiciliated cells; spermatogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

