New Perspectives in the Medical Treatment of Non-Muscle-Invasive Bladder Cancer: Immune Checkpoint Inhibitors and Beyond
- PMID: 35159167
- PMCID: PMC8834622
- DOI: 10.3390/cells11030357
New Perspectives in the Medical Treatment of Non-Muscle-Invasive Bladder Cancer: Immune Checkpoint Inhibitors and Beyond
Abstract
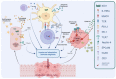
Non-muscle-invasive bladder cancer (NMIBC) is characterized by a high rate of cure, but also by a non-negligible probability of recurrence and risk progression to muscle-invasive disease. NMIBC management requires a proper local resection and staging, followed by a risk-based treatment with intravesical agents. For many years, the current gold standard treatment for patients with intermediate or high-risk disease is transurethral resection of the bladder (TURB) followed by intravesical bacillus Calmette-Guérin (BCG) instillations. Unfortunately, in about half of high-risk patients, intravesical BCG treatment fails and NMIBC persists or recurs early. While radical cystectomy remains the gold standard for these patients, new therapeutic targets are being individuated and studied. Radical cystectomy in fact can provide an excellent long-term disease control, but can deeply interfere with quality of life. In particular, the enhanced immune checkpoints expression shown in BCG-unresponsive patients and the activity of immune checkpoints inhibitors (ICIs) in advanced bladder cancer provided the rationale for testing ICIs in NMIBC. Recently, pembrolizumab has shown promising activity in BCG-unresponsive NMIBC patients, obtaining FDA approval. Meanwhile multiple novel drugs with alternative mechanisms of action have proven to be safe and effective in NMIBC treatment and others are under investigation. The aim of this review is to analyse and describe the clinical activity of new emerging drugs in BCG-unresponsive NMIBC focusing on immunotherapy results.
Keywords: BGC-unresponsive; immune-checkpoint inhibitors; immunotherapy; non-muscle-invasive bladder cancer; pembrolizumab.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Globocan IARC Cancer Today Bladder Cancer Fact Sheet. 2020. [(accessed on 31 December 2021)]. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf.
-
- Compérat E., Gontero P., Mostafid A.H., Palou J., Van Rhijn B.W.G., Rouprêt M., Shariat S.F., Sylvester R., Zigeuner R. Non-muscle-invasive Bladder Cancer (TaT1 and CIS) EAU Guidelines. Eur. Urol. 2019;31:1–48. - PubMed
-
- Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Dominguez Escrig J.L., Gontero P., Liedberg F., Masson-Lecomte A., Mostafid A.H., et al. European Association of Urology Guidelines on Non–muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ) Eur. Urol. 2021;81:75–94. doi: 10.1016/j.eururo.2021.08.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

