The Microbiota-Gut Axis in Premature Infants: Physio-Pathological Implications
- PMID: 35159189
- PMCID: PMC8834399
- DOI: 10.3390/cells11030379
The Microbiota-Gut Axis in Premature Infants: Physio-Pathological Implications
Abstract
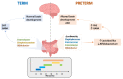
Intriguing evidence is emerging in regard to the influence of gut microbiota composition and function on host health from the very early stages of life. The development of the saprophytic microflora is conditioned by several factors in infants, and peculiarities have been found for babies born prematurely. This population is particularly exposed to a high risk of infection, postnatal antibiotic treatment, feeding difficulties and neurodevelopmental disabilities. To date, there is still a wide gap in understanding all the determinants and the mechanism behind microbiota disruption and its influence in the development of the most common complications of premature infants. A large body of evidence has emerged during the last decades showing the existence of a bidirectional communication axis involving the gut microbiota, the gut and the brain, defined as the microbiota-gut-brain axis. In this context, given that very few data are available to demonstrate the correlation between microbiota dysbiosis and neurodevelopmental disorders in preterm infants, increasing interest has arisen to better understand the impact of the microbiota-gut-brain axis on the clinical outcomes of premature infants and to clarify how this may lead to alternative preventive, diagnostic and therapeutic strategies. In this review, we explored the current evidence regarding microbiota development in premature infants, focusing on the effects of delivery mode, type of feeding, environmental factors and possible influence of the microbiota-gut-brain axis on preterm clinical outcomes during their hospital stay and on their health status later in life.
Keywords: gut-brain axis; microbiota; preterm infants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Allotey J., Zamora J., Cheong-See F., Kalidindi M., Arroyo-Manzano D., Asztalos E., van der Post J.A.M., Mol B.W., Moore D., Birtles D., et al. Cognitive, motor, behavioural and academic performances of children born preterm: A meta-analysis and systematic review involving 64,061 children. BJOG Int. J. Obstet. Gynaecol. 2018;125:16–25. doi: 10.1111/1471-0528.14832. - DOI - PubMed
-
- Pierrat V., Marchand-Martin L., Arnaud C., Kaminski M., Resche-Rigon M., Lebeaux C., Bodeau-Livinec F., Morgan A.S., Goffinet F., Marret S., et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:3448. doi: 10.1136/bmj.j3448. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

