Functional Differences between Proteasome Subtypes
- PMID: 35159231
- PMCID: PMC8834425
- DOI: 10.3390/cells11030421
Functional Differences between Proteasome Subtypes
Abstract
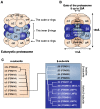
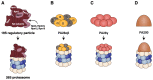
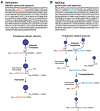
Four proteasome subtypes are commonly present in mammalian tissues: standard proteasomes, which contain the standard catalytic subunits β1, β2 and β5; immunoproteasomes containing the immuno-subunits β1i, β2i and β5i; and two intermediate proteasomes, containing a mix of standard and immuno-subunits. Recent studies revealed the expression of two tissue-specific proteasome subtypes in cortical thymic epithelial cells and in testes: thymoproteasomes and spermatoproteasomes. In this review, we describe the mechanisms that enable the ATP- and ubiquitin-dependent as well as the ATP- and ubiquitin-independent degradation of proteins by the proteasome. We focus on understanding the role of the different proteasome subtypes in maintaining protein homeostasis in normal physiological conditions through the ATP- and ubiquitin-dependent degradation of proteins. Additionally, we discuss the role of each proteasome subtype in the ATP- and ubiquitin-independent degradation of disordered proteins. We also discuss the role of the proteasome in the generation of peptides presented by MHC class I molecules and the implication of having different proteasome subtypes for the peptide repertoire presented at the cell surface. Finally, we discuss the role of the immunoproteasome in immune cells and its modulation as a potential therapy for autoimmune diseases.
Keywords: ATP- and ubiquitin-dependent degradation; ATP- and ubiquitin-independent degradation; MHC class I peptides; autoimmune diseases; proteasome subtypes; protein degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

