Inhibition of Nigral Microglial Activation Reduces Age-Related Loss of Dopaminergic Neurons and Motor Deficits
- PMID: 35159290
- PMCID: PMC8834087
- DOI: 10.3390/cells11030481
Inhibition of Nigral Microglial Activation Reduces Age-Related Loss of Dopaminergic Neurons and Motor Deficits
Abstract
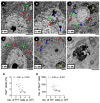
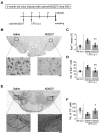
Parkinson's disease (PD) is an age-related neurodegenerative disease caused by a selective loss of dopaminergic (DA) neurons in the substantia nigra (SN). Microglial activation is implicated in the pathogenesis of PD. This study aimed to characterize the role of microglial activation in aging-related nigral DA neuron loss and motor deficits in mice. We showed that, compared to 3-month-old mice, the number of DA neurons in the SN and the expression of dopamine transporter (DAT) in the striatum decreased during the period of 9 to 12 months of age. Motor deficits and microglial activation in the SN were also evident during these months. The number of DA neurons was negatively correlated with the degrees of microglial activation. The inhibition of age-related microglial activation by ibuprofen during these 3 months decreased DA neuron loss in the SN. Eliminating the microglia prevented systemic inflammation-induced DA neuron death. Forcing mice to run during these 3 months inhibited microglial activation and DA neuron loss. Blocking the brain-derived neurotrophic factor (BDNF) signaling eliminated the exercise-induced protective effects. In conclusion, nigral DA neurons were susceptible to local microglial activation. Running exercise upregulated BDNF-TrkB signaling and inhibited microglial activation during aging. Long-term exercise can be considered as a non-pharmacological strategy to ameliorate microglial activation and related neurodegeneration.
Keywords: brain-derived neurotrophic factor; dopaminergic neurons; exercise; microglial activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

