GPR183 Is Dispensable for B1 Cell Accumulation and Function, but Affects B2 Cell Abundance, in the Omentum and Peritoneal Cavity
- PMID: 35159303
- PMCID: PMC8834096
- DOI: 10.3390/cells11030494
GPR183 Is Dispensable for B1 Cell Accumulation and Function, but Affects B2 Cell Abundance, in the Omentum and Peritoneal Cavity
Abstract
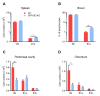
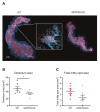
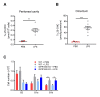
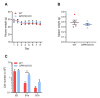
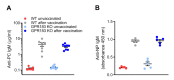
B1 cells constitute a specialized subset of B cells, best characterized in mice, which is abundant in body cavities, including the peritoneal cavity. Through natural and antigen-induced antibody production, B1 cells participate in the early defense against bacteria. The G protein-coupled receptor 183 (GPR183), also known as Epstein-Barr virus-induced gene 2 (EBI2), is an oxysterol-activated chemotactic receptor that regulates migration of B cells. We investigated the role of GPR183 in B1 cells in the peritoneal cavity and omentum. B1 cells expressed GPR183 at the mRNA level and migrated towards the GPR183 ligand 7α,25-dihydroxycholesterol (7α,25-OHC). GPR183 knock-out (KO) mice had smaller omenta, but with normal numbers of B1 cells, whereas they had fewer B2 cells in the omentum and peritoneal cavity than wildtype (WT) mice. GPR183 was not responsible for B1 cell accumulation in the omentum in response to i.p. lipopolysaccharide (LPS)-injection, in spite of a massive increase in 7α,25-OHC levels. Lack of GPR183 also did not affect B1a- or B1b cell-specific antibody responses after vaccination. In conclusion, we found that GPR183 is non-essential for the accumulation and function of B1 cells in the omentum and peritoneal cavity, but that it influences the abundance of B2 cells in these compartments.
Keywords: 7TM receptor; B-1 cell; B1 cell; EBI2; GPCR; GPR183; oxysterol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

