Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders
- PMID: 35159304
- PMCID: PMC8834275
- DOI: 10.3390/cells11030495
Pregnant Women and Endocrine Disruptors: Role of P2X7 Receptor and Mitochondrial Alterations in Placental Cell Disorders
Abstract
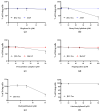
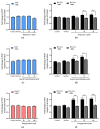
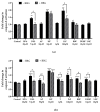
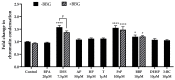
In pregnant women, the lungs, skin and placenta are exposed daily to endocrine-disrupting chemicals (EDCs). EDCs induce multiple adverse effects, not only on endocrine organs, but also on non-endocrine organs, with the P2X7 cell death receptor being potentially the common key element. Our objective was first to investigate mechanisms of EDCs toxicity in both endocrine and non-endocrine cells through P2X7 receptor activation, and second, to compare the level of activation in lung, skin and placental cells. In addition, apoptosis in placental cells was studied because the placenta is the most exposed organ to EDCs and has essential endocrine functions. A total of nine EDCs were evaluated on three human cell models. We observed that the P2X7 receptor was not activated by EDCs in lung non-endocrine cells but was activated in skin and placenta cells, with the highest activation in placenta cells. P2X7 receptor activation and apoptosis are pathways shared by all tested EDCs in endocrine placental cells. P2X7 receptor activation along with apoptosis induction could be key elements in understanding endocrine placental and skin disorders induced by EDCs.
Keywords: P2X7 receptor; apoptosis; endocrine disruptors; lung toxicity; mitochondrial alterations; placental toxicity; skin toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Cocktail Effect of Endocrine Disrupting Chemicals: Application to Chlorpyrifos in Lavender Essential Oils.Int J Environ Res Public Health. 2022 Oct 10;19(19):12984. doi: 10.3390/ijerph191912984. Int J Environ Res Public Health. 2022. PMID: 36232284 Free PMC article.
-
Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy.Trends Endocrinol Metab. 2020 Jul;31(7):508-524. doi: 10.1016/j.tem.2020.03.003. Epub 2020 Apr 2. Trends Endocrinol Metab. 2020. PMID: 32249015 Free PMC article. Review.
-
The effect of endocrine-disrupting chemicals on placental development.Front Endocrinol (Lausanne). 2023 Feb 21;14:1059854. doi: 10.3389/fendo.2023.1059854. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36896182 Free PMC article. Review.
-
A mechanism for the effect of endocrine disrupting chemicals on placentation.Chemosphere. 2019 Sep;231:326-336. doi: 10.1016/j.chemosphere.2019.05.133. Epub 2019 May 18. Chemosphere. 2019. PMID: 31132539 Review.
-
Occurrence, placental transfer, and health risks of emerging endocrine-disrupting chemicals in pregnant women.J Hazard Mater. 2023 Oct 5;459:132157. doi: 10.1016/j.jhazmat.2023.132157. Epub 2023 Jul 26. J Hazard Mater. 2023. PMID: 37506642
Cited by
-
Metabolic Inflammation and Cellular Immunity.Cells. 2023 Jun 13;12(12):1615. doi: 10.3390/cells12121615. Cells. 2023. PMID: 37371085 Free PMC article.
-
Endocrine Disruptor Compounds in Environment: Focus on Women's Reproductive Health and Endometriosis.Int J Mol Sci. 2023 Mar 16;24(6):5682. doi: 10.3390/ijms24065682. Int J Mol Sci. 2023. PMID: 36982755 Free PMC article. Review.
-
Cocktail Effect of Endocrine Disrupting Chemicals: Application to Chlorpyrifos in Lavender Essential Oils.Int J Environ Res Public Health. 2022 Oct 10;19(19):12984. doi: 10.3390/ijerph191912984. Int J Environ Res Public Health. 2022. PMID: 36232284 Free PMC article.
-
Maternal Serum, Cord and Human Milk Levels of Per- and Polyfluoroalkyl Substances (PFAS), Association with Predictors and Effect on Newborn Anthropometry.Toxics. 2023 May 14;11(5):455. doi: 10.3390/toxics11050455. Toxics. 2023. PMID: 37235269 Free PMC article.
-
Forskolin Induces Endocrine Disturbance in Human JEG-3 Placental Cells.Toxics. 2022 Jun 30;10(7):355. doi: 10.3390/toxics10070355. Toxics. 2022. PMID: 35878261 Free PMC article.
References
-
- IPCS . Global Assessment of the State-of-the-Science of Endocrine Disruptor. World Health Organization; Geneva, Switzerland: 2002.
-
- Chen X., Chen M., Xu B., Tang R., Han X., Qin Y., Xu B., Hang B., Mao Z., Huo W., et al. Parental phenols exposure and spontaneous abortion in Chinese population residing in the middle and lower reaches of the Yangtze River. Chemosphere. 2013;93:217–222. doi: 10.1016/j.chemosphere.2013.04.067. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

