Intestinal Microbiota Remodeling Protects Mice from Western Diet-Induced Brain Inflammation and Cognitive Decline
- PMID: 35159313
- PMCID: PMC8834507
- DOI: 10.3390/cells11030504
Intestinal Microbiota Remodeling Protects Mice from Western Diet-Induced Brain Inflammation and Cognitive Decline
Abstract
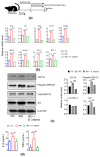
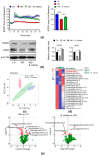
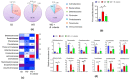
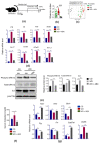
It has been shown that the Western diet (WD) induces systemic inflammation and cognitive decline. Moreover, probiotic supplementation and antibiotic treatment reduce diet-induced hepatic inflammation. The current study examines whether shaping the gut microbes by Bifidobacterium infantis (B. infantis) supplementation and antibiotic treatment reduce diet-induced brain inflammation and improve neuroplasticity. Furthermore, the significance of bile acid (BA) signaling in regulating brain inflammation was studied. Mice were fed a control diet (CD) or WD for seven months. B. infantis was supplemented to WD-fed mice to study brain inflammation, lipid, metabolomes, and neuroplasticity measured by long-term potentiation (LTP). Broad-spectrum coverage antibiotics and cholestyramine treatments were performed to study the impact of WD-associated gut microbes and BA in brain inflammation. Probiotic B. infantis supplementation inhibited diet-induced brain inflammation by reducing IL6, TNFα, and CD11b levels. B. infantis improved LTP and increased brain PSD95 and BDNF levels, which were reduced due to WD intake. Additionally, B. infantis reduced cecal cholesterol, brain ceramide and enhanced saturated fatty acids. Moreover, antibiotic treatment, as well as cholestyramine, diminished WD-induced brain inflammatory signaling. Our findings support the theory that intestinal microbiota remodeling by B. infantis reduces brain inflammation, activates BA receptor signaling, and improves neuroplasticity.
Keywords: Bifidobacterium infantis; bile acid receptor; brain inflammation; gut microbiota; metabolomics; neuroplasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Jena P.K., Sheng L., Di Lucente J., Jin L.W., Maezawa I., Wan Y.Y. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J. 2018;32:2866–2877. doi: 10.1096/fj.201700984RR. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

