Induction of Pro-Fibrotic CLIC4 in Dermal Fibroblasts by TGF-β/Wnt3a Is Mediated by GLI2 Upregulation
- PMID: 35159339
- PMCID: PMC8834396
- DOI: 10.3390/cells11030530
Induction of Pro-Fibrotic CLIC4 in Dermal Fibroblasts by TGF-β/Wnt3a Is Mediated by GLI2 Upregulation
Abstract
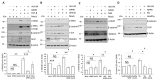
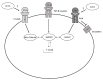
Chloride intracellular channel 4 (CLIC4) is a recently discovered driver of fibroblast activation in Scleroderma (SSc) and cancer-associated fibroblasts (CAF). CLIC4 expression and activity are regulated by TGF-β signalling through the SMAD3 transcription factor. In view of the aberrant activation of canonical Wnt-3a and Hedgehog (Hh) signalling in fibrosis, we investigated their role in CLIC4 upregulation. Here, we show that TGF-β/SMAD3 co-operates with Wnt3a/β-catenin and Smoothened/GLI signalling to drive CLIC4 expression in normal dermal fibroblasts, and that the inhibition of β-catenin and GLI expression or activity abolishes TGF-β/SMAD3-dependent CLIC4 induction. We further show that the expression of the pro-fibrotic marker α-smooth muscle actin strongly correlates with CLIC4 expression in dermal fibroblasts. Further investigations revealed that the inhibition of CLIC4 reverses morphogen-dependent fibroblast activation. Our data highlights that CLIC4 is a common downstream target of TGF-β, Hh, and Wnt-3a through signalling crosstalk and we propose a potential therapeutic avenue using CLIC4 inhibitors.
Keywords: CLIC4; Ion channels; Scleroderma; fibrosis; morphogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mao D.Y., Kleinjan M.L., Jilishitz I., Swaminathan B., Obinata H., Komarova Y.A., Bayless K.J., Hla T., Kitajewski J.K. CLIC1 and CLIC4 mediate endothelial S1P receptor signalling to facilitate Rac1 and RhoA activity and function. Sci. Signal. 2021;14:eabc0425. doi: 10.1126/scisignal.abc0425. - DOI - PMC - PubMed
-
- Fernández-Salas E., Suh K.S., Speransky V.V., Bowers W.L., Levy J.M., Adams T., Pathak K.R., Edwards L.E., Hayes D.D., Cheng C., et al. mtCLIC/CLIC4, an organellular chloride channel protein is increased by DNA damage and participates in the apoptotic response to p53. Mol. Cell. Biol. 2002;22:3610–3620. doi: 10.1128/MCB.22.11.3610-3620.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

