Cell Wall Properties Determine Genotype-Specific Response to Cold in Miscanthus × giganteus Plants
- PMID: 35159356
- PMCID: PMC8834381
- DOI: 10.3390/cells11030547
Cell Wall Properties Determine Genotype-Specific Response to Cold in Miscanthus × giganteus Plants
Abstract
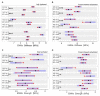
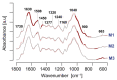
The cell wall plays a crucial role in plant growth and development, including in response to environmental factors, mainly through significant biochemical and biomechanical plasticity. The involvement of the cell wall in C4 plants' response to cold is, however, still poorly understood. Miscanthus × giganteus, a perennial grass, is generally considered cold tolerant and, in contrast to other thermophilic species such as maize or sorgo, can maintain a relatively high level of photosynthesis efficiency at low ambient temperatures. This unusual response to chilling among C4 plants makes Miscanthus an interesting study object in cold acclimation mechanism research. Using the results obtained from employing a diverse range of techniques, including analysis of plasmodesmata ultrastructure by means of transmission electron microscopy (TEM), infrared spectroscopy (FTIR), and biomechanical tests coupled with photosynthetic parameters measurements, we present evidence for the implication of the cell wall in genotype-specific responses to cold in this species. The observed reduction in the assimilation rate and disturbance of chlorophyll fluorescence parameters in the susceptible M3 genotype under cold conditions were associated with changes in the ultrastructure of the plasmodesmata, i.e., a constriction of the cytoplasmic sleeve in the central region of the microchannel at the mesophyll-bundle sheath interface. Moreover, this cold susceptible genotype was characterized by enhanced tensile stiffness, strength of leaf wall material, and a less altered biochemical profile of the cell wall, revealed by FTIR spectroscopy, compared to cold tolerant genotypes. These changes indicate that a decline in photosynthetic activity may result from a decrease in leaf CO2 conductance due to the formation of more compact and thicker cell walls and that an enhanced tolerance to cold requires biochemical wall remodelling. Thus, the well-established trade-off between photosynthetic capacity and leaf biomechanics found across multiple species in ecological research may also be a relevant factor in Miscanthus' tolerance to cold. In this paper, we demonstrate that M. giganteus genotypes showing a high degree of genetic similarity may respond differently to cold stress if exposed at earlier growing seasons to various temperature regimes, which has implications for the cell wall modifications patterns.
Keywords: C4 plants; FTIR spectroscopy; Miscanthus × giganteus; biomechanical tests; cell wall; cold tolerance; photosynthetic activity; plasmodesmata.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

