Mechanisms of Cisplatin Resistance in HPV Negative Head and Neck Squamous Cell Carcinomas
- PMID: 35159370
- PMCID: PMC8834318
- DOI: 10.3390/cells11030561
Mechanisms of Cisplatin Resistance in HPV Negative Head and Neck Squamous Cell Carcinomas
Abstract
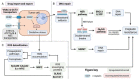
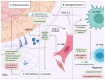
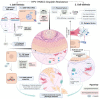
Head and neck squamous cell carcinomas (HNSCCs) are the eighth most common cancers worldwide. While promising new therapies are emerging, cisplatin-based chemotherapy remains the gold standard for advanced HNSCCs, although most of the patients relapse due to the development of resistance. This review aims to condense the different mechanisms involved in the development of cisplatin resistance in HNSCCs and highlight future perspectives intended to overcome its related complications. Classical resistance mechanisms include drug import and export, DNA repair and oxidative stress control. Emerging research identified the prevalence of these mechanisms in populations of cancer stem cells (CSC), which are the cells mainly contributing to cisplatin resistance. The use of old and new CSC markers has enabled the identification of the characteristics within HNSCC CSCs predisposing them to treatment resistance, such as cell quiescence, increased self-renewal capacity, low reactive oxygen species levels or the acquisition of epithelial to mesenchymal transcriptional programs. In the present review, we will discuss how cell intrinsic and extrinsic cues alter the phenotype of CSCs and how they influence resistance to cisplatin treatment. In addition, we will assess how the stromal composition and the tumor microenvironment affect drug resistance and the acquisition of CSCs' characteristics through a complex interplay between extracellular matrix content as well as immune and non-immune cell characteristics. Finally, we will describe how alterations in epigenetic modifiers or other signaling pathways can alter tumor behavior and cell plasticity to induce chemotherapy resistance. The data generated in recent years open up a wide range of promising strategies to optimize cisplatin therapy, with the potential to personalize HNSCC patient treatment strategies.
Keywords: HNSCC; cancer stem cells; cell plasticity; cisplatin; epigenetics; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- International Agency for Research on Cancer List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans, Volumes 1 to 127. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. 2020. [(accessed on 1 February 2022)]. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

