Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells
- PMID: 35159379
- PMCID: PMC8834431
- DOI: 10.3390/cells11030570
Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells
Abstract
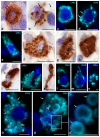
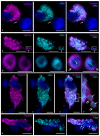
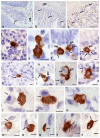
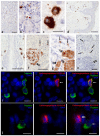
Carboxypeptidase A3 (CPA3) is a specific mast cell (MC) protease with variable expression. This protease is one of the preformed components of the secretome. During maturation of granules, CPA3 becomes an active enzyme with a characteristic localization determining the features of the cytological and ultrastructural phenotype of MC. CPA3 takes part in the regulation of a specific tissue microenvironment, affecting the implementation of innate immunity, the mechanisms of angiogenesis, the processes of remodeling of the extracellular matrix, etc. Characterization of CPA3 expression in MC can be used to refine the MC classification, help in a prognosis, and increase the effectiveness of targeted therapy.
Keywords: carboxypeptidase A3; granules; mast cells; secretome; secretory pathways; specific tissue microenvironment.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Crivellato E., Travan L., Ribatti D. The phylogenetic profile of mast cells. Methods Mol. Biol. 2015;1220:11–27. - PubMed
-
- Valent P., Akin C., Hartmann K., Nilsson G., Reiter A., Hermine O., Sotlar K., Sperr W.R., Escribano L., George T.I., et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics. 2020;10:10743–10768. doi: 10.7150/thno.46719. - DOI - PMC - PubMed
-
- Pejler G., Abrink M., Ringvall M., Wernersson S. Mast cell proteases. Adv. Immunol. 2007;95:167–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

