Regulation of Nucleolar Activity by MYC
- PMID: 35159381
- PMCID: PMC8834138
- DOI: 10.3390/cells11030574
Regulation of Nucleolar Activity by MYC
Abstract
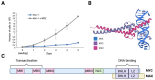
The nucleolus harbors the machinery necessary to produce new ribosomes which are critical for protein synthesis. Nucleolar size, shape, and density are highly dynamic and can be adjusted to accommodate ribosome biogenesis according to the needs for protein synthesis. In cancer, cells undergo continuous proliferation; therefore, nucleolar activity is elevated due to their high demand for protein synthesis. The transcription factor and universal oncogene MYC promotes nucleolar activity by enhancing the transcription of ribosomal DNA (rDNA) and ribosomal proteins. This review summarizes the importance of nucleolar activity in mammalian cells, MYC's role in nucleolar regulation in cancer, and discusses how a better understanding (and the potential inhibition) of aberrant nucleolar activity in cancer cells could lead to novel therapeutics.
Keywords: MYC; cell growth; nucleolus; ribosome; ribosome biogenesis; translation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

