Roles of Podoplanin in Malignant Progression of Tumor
- PMID: 35159384
- PMCID: PMC8834262
- DOI: 10.3390/cells11030575
Roles of Podoplanin in Malignant Progression of Tumor
Abstract
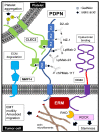
Podoplanin (PDPN) is a cell-surface mucin-like glycoprotein that plays a critical role in tumor development and normal development of the lung, kidney, and lymphatic vascular systems. PDPN is overexpressed in several tumors and is involved in their malignancy. PDPN induces platelet aggregation through binding to platelet receptor C-type lectin-like receptor 2. Furthermore, PDPN modulates signal transductions that regulate cell proliferation, differentiation, migration, invasion, epithelial-to-mesenchymal transition, and stemness, all of which are crucial for the malignant progression of tumor. In the tumor microenvironment (TME), PDPN expression is upregulated in the tumor stroma, including cancer-associated fibroblasts (CAFs) and immune cells. CAFs play significant roles in the extracellular matrix remodeling and the development of immunosuppressive TME. Additionally, PDPN functions as a co-inhibitory molecule on T cells, indicating its involvement with immune evasion. In this review, we describe the mechanistic basis and diverse roles of PDPN in the malignant progression of tumors and discuss the possibility of the clinical application of PDPN-targeted cancer therapy, including cancer-specific monoclonal antibodies, and chimeric antigen receptor T technologies.
Keywords: CasMab; PDPN; antibody therapy; cancer-specific monoclonal antibody; podoplanin; tumor malignancy; tumor marker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Link between tumor-promoting fibrous microenvironment and an immunosuppressive microenvironment in stage I lung adenocarcinoma.Lung Cancer. 2018 Dec;126:64-71. doi: 10.1016/j.lungcan.2018.10.021. Epub 2018 Oct 28. Lung Cancer. 2018. PMID: 30527194
-
Relationship between podoplanin-expressing cancer-associated fibroblasts and the immune microenvironment of early lung squamous cell carcinoma.Lung Cancer. 2021 Mar;153:1-10. doi: 10.1016/j.lungcan.2020.12.020. Epub 2020 Dec 24. Lung Cancer. 2021. PMID: 33429158
-
CAF Specific Expression of Podoplanin May Be Dispensable for the Malignancy of Malignant Melanoma.Mol Carcinog. 2025 Feb;64(2):215-220. doi: 10.1002/mc.23841. Epub 2024 Nov 8. Mol Carcinog. 2025. PMID: 39513649 Free PMC article.
-
Podoplanin: An emerging cancer biomarker and therapeutic target.Cancer Sci. 2018 May;109(5):1292-1299. doi: 10.1111/cas.13580. Cancer Sci. 2018. PMID: 29575529 Free PMC article. Review.
-
Platelet CLEC2-Podoplanin Axis as a Promising Target for Oral Cancer Treatment.Front Immunol. 2021 Dec 15;12:807600. doi: 10.3389/fimmu.2021.807600. eCollection 2021. Front Immunol. 2021. PMID: 34987523 Free PMC article. Review.
Cited by
-
A novel PDPN antagonist peptide CY12-RP2 inhibits melanoma growth via Wnt/β-catenin and modulates the immune cells.J Exp Clin Cancer Res. 2024 Jan 2;43(1):9. doi: 10.1186/s13046-023-02910-y. J Exp Clin Cancer Res. 2024. PMID: 38167452 Free PMC article.
-
Functional analysis of fibroblasts and macrophages in head and neck paragangliomas.Front Endocrinol (Lausanne). 2024 Nov 15;15:1397839. doi: 10.3389/fendo.2024.1397839. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39619326 Free PMC article.
-
Therapeutic Apheresis Using a β2-Microglobulin Removal Column Reduces Circulating Tumor Cell Count.J Pers Med. 2024 Jun 15;14(6):640. doi: 10.3390/jpm14060640. J Pers Med. 2024. PMID: 38929860 Free PMC article.
-
Therapeutic Strategies Focused on Cancer-Associated Hypercoagulation for Ovarian Clear Cell Carcinoma.Cancers (Basel). 2022 Apr 24;14(9):2125. doi: 10.3390/cancers14092125. Cancers (Basel). 2022. PMID: 35565252 Free PMC article. Review.
-
Lectin Receptors in Primary and Metastatic Cancer Cells.Asian Pac J Cancer Prev. 2024 Nov 1;25(11):4027-4034. doi: 10.31557/APJCP.2024.25.11.4027. Asian Pac J Cancer Prev. 2024. PMID: 39611927 Free PMC article.
References
-
- Takei J., Itai S., Harada H., Furusawa Y., Miwa T., Fukui M., Nakamura T., Sano M., Sayama Y., Yanaka M., et al. Characterization of Anti-Goat Podoplanin Monoclonal Antibody PMab-235 Using Immunohistochemistry Against Goat Tissues. Monoclon. Antib. Immunodiagn. Immunother. 2019;38:213–219. doi: 10.1089/mab.2019.0022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

