The Effect of High Protein Powder Structure on Hydration, Glass Transition, Water Sorption, and Thermomechanical Properties
- PMID: 35159444
- PMCID: PMC8834494
- DOI: 10.3390/foods11030292
The Effect of High Protein Powder Structure on Hydration, Glass Transition, Water Sorption, and Thermomechanical Properties
Abstract
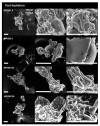
Poor solubility of high protein milk powders can be an issue during the production of nutritional formulations, as well as for end-users. One possible way to improve powder solubility is through the creation of vacuoles and pores in the particle structure using high pressure gas injection during spray drying. The aim of this study was to determine whether changes in particle morphology effect physical properties, such as hydration, water sorption, structural strength, glass transition temperature, and α-relaxation temperatures. Four milk protein concentrate powders (MPC, 80%, w/w, protein) were produced, i.e., regular (R) and agglomerated (A) without nitrogen injection and regular (RN) and agglomerated (AN) with nitrogen injection. Electron microscopy confirmed that nitrogen injection increased powder particles' sphericity and created fractured structures with pores in both regular and agglomerated systems. Environmental scanning electron microscopy (ESEM) showed that nitrogen injection enhanced the moisture uptake and solubility properties of RN and AN as compared with non-nitrogen-injected powders (R and A). In particular, at the final swelling at over 100% relative humidity (RH), R, A, AN, and RN powders showed an increase in particle size of 25, 20, 40, and 97% respectively. The injection of nitrogen gas (NI) did not influence calorimetric glass transition temperature (Tg), which could be expected as there was no change to the powder composition, however, the agglomeration of powders did effect Tg. Interestingly, the creation of porous powder particles by NI did alter the α-relaxation temperatures (up to ~16 °C difference between R and AN powders at 44% RH) and the structural strength (up to ~11 °C difference between R and AN powders at 44% RH). The results of this study provide an in-depth understanding of the changes in the morphology and physical-mechanical properties of nitrogen gas-injected MPC powders.
Keywords: dynamic mechanical analysis (DMA); environmental scanning electron microscope (ESEM); gas injection; glass transition; milk protein concentrate (MPC); structural strength; α-relaxation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Baldwin A.J., Truong G.N.T. Development of insolubility in dehydration of dairy milk powders. Food Bioprod. Process. 2007;85:202–208. doi: 10.1205/fbp07008. - DOI
-
- Jimenez-Flores R., Kosikowski F.V. Properties of ultrafiltered skim milk retentate powders. J. Dairy Sci. 1986;69:329–339. doi: 10.3168/jds.S0022-0302(86)80410-6. - DOI
-
- Schuck P., Piot M., Méjean S., Fauquant J., Brulé G., Maubois J.L. Dehydration of an ultra-clean milk and micellar casein enriched milks. Lait. 1994;74:47–63. doi: 10.1051/lait:199415. - DOI
-
- Dybing S.T., Bhaskar G.V., Dunlop F.P., Fayerman A.M., Whitton M.J. Modified Milk Protein Concentrates and Their Use in Making Gels and Dairy Products. 7,192,619. U.S. Patent. 2007 March 20;
-
- Anema S.G., Pinder D.N., Hunter R.J., Hemar Y. Effects of storage temperature on the solubility of milk protein concentrate (MPC85) Food Hydrocoll. 2006;20:386–393. doi: 10.1016/j.foodhyd.2005.03.015. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

