Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis
- PMID: 35159969
- PMCID: PMC8836604
- DOI: 10.3390/jcm11030517
Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis
Abstract
Background: Airway stent implantation used in the treatment of benign cicatricial airway stenosis (BCAS) can lead to local granulation and scar formation, resulting in restenosis and treatment failure.
Methods: We systematically investigated a paclitaxel-loaded PLGA-coating stent (PLPCS) and analyzed the safety and efficacy of the PLPCS in patients with BCAS. Patients were enrolled from four hospitals in China and observed for six months after implantation, by bronchoscopy performed weekly in the first month and monthly thereafter. The stent was removed immediately upon detection of granulation tissue proliferation, leading to immobility of the stent.
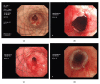
Results: Granulation tissue was formed one week after stent implantation, most of which was located at the upper edge of the stent and the narrowest airway in the stent. All stents were removed in three months (mean: 6.51 + 4.67 weeks), with a curative outcome in one case and ineffective results in two. The remaining seven patients developed complications within three months, necessitating early stent removal. The main complication was granulation formation, resulting in difficulty in stent removal.
Conclusion: Although PLPCS showed beneficial effects in basic and animal experiments, it cannot prevent airway restenosis in actual practice, mainly due to granulation formation.
Keywords: benign airway stenosis; paclitaxel; stent; treatment.
Conflict of interest statement
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
Figures

Similar articles
-
Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis.Respiration. 2017;93(6):430-435. doi: 10.1159/000472155. Epub 2017 Apr 28. Respiration. 2017. PMID: 28448981
-
[Airway metal stents removal by rigid bronchoscopy].Zhonghua Jie He He Hu Xi Za Zhi. 2016 Feb;39(2):98-104. doi: 10.3760/cma.j.issn.1001-0939.2016.02.005. Zhonghua Jie He He Hu Xi Za Zhi. 2016. PMID: 26879612 Chinese.
-
Paclitaxel Drug-eluting Tracheal Stent Could Reduce Granulation Tissue Formation in a Canine Model.Chin Med J (Engl). 2016 Nov 20;129(22):2708-2713. doi: 10.4103/0366-6999.193461. Chin Med J (Engl). 2016. PMID: 27824004 Free PMC article.
-
Drug-eluting stents.Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review.
-
Expandable stents.Chest Surg Clin N Am. 1996 May;6(2):305-28. Chest Surg Clin N Am. 1996. PMID: 8724281 Review.
Cited by
-
Solubilization of Paclitaxel with Natural Compound Rubusoside toward Improving Oral Bioavailability in a Rodent Model.Pharmaceutics. 2024 Aug 22;16(8):1104. doi: 10.3390/pharmaceutics16081104. Pharmaceutics. 2024. PMID: 39204449 Free PMC article.
-
pH-Responsive Nanoparticles for Delivery of Paclitaxel to the Injury Site for Inhibiting Vascular Restenosis.Pharmaceutics. 2022 Feb 27;14(3):535. doi: 10.3390/pharmaceutics14030535. Pharmaceutics. 2022. PMID: 35335910 Free PMC article.
-
Analysis of clinical characteristics of 617 patients with benign airway stenosis.Front Med (Lausanne). 2023 Jul 20;10:1202309. doi: 10.3389/fmed.2023.1202309. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37547601 Free PMC article.
-
Synergistic effect of VEGF and SDF-1α in endothelial progenitor cells and vascular smooth muscle cells.Front Pharmacol. 2022 Jul 15;13:914347. doi: 10.3389/fphar.2022.914347. eCollection 2022. Front Pharmacol. 2022. PMID: 35910392 Free PMC article.
-
Advances in studies on tracheal stent design addressing the related complications.Mater Today Bio. 2024 Sep 21;29:101263. doi: 10.1016/j.mtbio.2024.101263. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39399242 Free PMC article. Review.
References
-
- Tsakiridis K., Darwiche K., Visouli A.N., Zarogoulidis P., Machairiotis N., Christofis C., Stylianaki A., Katsikogiannis N., Mpakas A., Courcoutsakis N., et al. Management of complex benign post-tracheostomy tracheal stenosis with bronchoscopic insertion of silicon tracheal stents, in patients with failed or contraindicated surgical reconstruction of trachea. J. Thorac. Dis. 2012;4((Suppl. S1)):32–40. doi: 10.3978/j.issn.207a439.2012.s002. - DOI - PMC - PubMed
-
- Singh T., Sandulache V.C., Otteson T.D., Barsic M., Klein E.C., Dohar J.E., Hebda P.A. Subglottic stenosis examined as a fibrotic response to airway injury characterized by altered mucosal fibroblast activity. Arch. Otolaryngol. Head Neck Surg. 2010;136:163–170. doi: 10.1001/archoto.2009.175. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

