Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis
- PMID: 35160031
- PMCID: PMC8836975
- DOI: 10.3390/jcm11030578
Multimodality Cardiac Imaging in Cardiomyopathies: From Diagnosis to Prognosis
Abstract
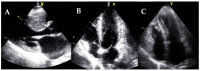
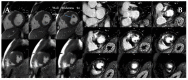
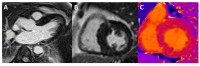
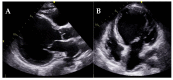
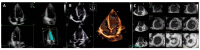
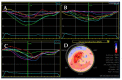
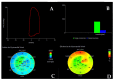
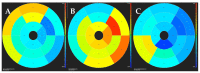
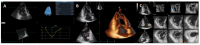
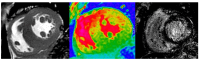
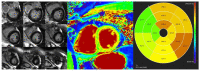
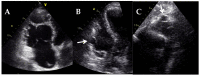
Cardiomyopathies are a group of structural and/or functional myocardial disorders which encompasses hypertrophic, dilated, arrhythmogenic, restrictive, and other cardiomyopathies. Multimodality cardiac imaging techniques are the cornerstone of cardiomyopathy diagnosis; transthoracic echocardiography should be the first-line imaging modality due to its availability, and diagnosis should be confirmed by cardiovascular magnetic resonance, which will provide more accurate morphologic and functional information, as well as extensive tissue characterization. Multimodality cardiac imaging techniques are also essential in assessing the prognosis of patients with cardiomyopathies; left ventricular ejection fraction and late gadolinium enhancement are two of the main variables used for risk stratification, and they are incorporated into clinical practice guidelines. Finally, periodic testing with cardiac imaging techniques should also be performed due to the evolving and progressive natural history of most cardiomyopathies.
Keywords: cardiomyopathy; diagnosis; late gadolinium enhancement; left ventricular ejection fraction; multimodality cardiac imaging techniques; prognosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Elliott P., Andersson B., Arbustini E., Bilinska Z., Cecchi F., Charron P., Dubourg O., Kühl U., Maisch B., McKenna W.J., et al. Classification of the cardiomyopathies: A position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. - DOI - PubMed
-
- Elliott P.M., Anastasakis A., Borger M.A., Borggrefe M., Cecchi F., Charron P., Hagege A.A., Lafont A., Limongelli G., Mahrholdt H., et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC) Eur. Heart J. 2014;35:2733–2779. - PubMed
-
- Ommen S.R., Mital S., Burke M.A., Day S.M., Deswal A., Elliott P., Evanovich L.L., Hung J., Joglar J.A., Kantor P., et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2020;76:e159–e240. - PubMed
-
- Cardim N., Galderisi M., Edvardsen T., Plein S., Popescu B.A., D’Andrea A., Bruder O., Cosyns B., Davin L., Donal E., et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: An expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur. Hear. J.-Cardiovasc. Imaging. 2015;16:280. doi: 10.1093/ehjci/jeu291. - DOI - PubMed
-
- Urbano-Moral J.A., Gutierrez-Garcia-Moreno L., Matabuena-Gomez-Limon J., Niella N., Maldonado G., Valle-Racero J.I., Niella M., Teixido-Tura G., Garcia-Dorado D., Ferrazzi P., et al. Structural abnormalities in hypertrophic cardiomyopathy beyond left ventricular hypertrophy by multimodality imaging evaluation. Echocardiography. 2019;36:1241–1252. doi: 10.1111/echo.14393. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

