Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study
- PMID: 35160074
- PMCID: PMC8837069
- DOI: 10.3390/jcm11030621
Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study
Abstract
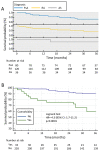
We evaluated the 3-year drug survival and efficacy of the biosimilar SB4/Benepali in rheumatoid arthritis (RA), psoriatic arthritis (PsA) and ankylosing spondylitis (AS) patients, previously treated with etanercept (ETA). Drug survival rate was calculated using the Kaplan-Meier method and Cox proportional hazard models were developed to examine predictors of SB4 discontinuation. 236 patients (120 RA, 80 PsA and 36 AS), aged 60.7 ± 13.8 years and with an ETA duration of 4.1 ± 3.4 years were included. The 3-year retention rate for SB4 was 94.4%, 88% and 86% in AS, RA and PsA patients, respectively, with no difference between groups. Patients without comorbid disease had higher retention rates vs. patients with comorbid disease (90% vs. 60%, p < 0.0001). Disease activity, as measured by DAS28, DAPSA and BASDAI remained stable over the 3 years. Comorbid disease (hazard ratio; HR: 4.06, p < 0.0001) and HAQ at baseline (HR: 2.42, p = 0.0024) significantly increased the risk of SB4 discontinuation, while previous ETA duration was negatively associated with SB4 discontinuation (HR: 0.97, p = 0.0064). Forty-one (17.4%) patients left the study due to the interruption of the SB4 treatment, 31 (75.6%) discontinued due to inefficacy and 10 (24.4%) due to adverse events. This real-life study confirms the similar efficacy profile of ETA with long-term retention and a good safety profile in inflammatory arthritis patients.
Keywords: SB4; anti-TNF; biosimilar; drug survival; inflammatory arthritis.
Conflict of interest statement
All authors have no conflict of interest to declare.
Figures



References
-
- Smolen J.S., Braun J., Dougados M., Emery P., Fitzgerald O., Helliwell P., Kavanaugh A., Kvien T.K., Landewé R., Luger T., et al. Treating Spondyloarthritis, Including Ankylosing Spondylitis and Psoriatic Arthritis, to Target: Recommendations of an International Task Force. Ann. Rheum. Dis. 2014;73:6–16. doi: 10.1136/annrheumdis-2013-203419. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

