Non-Interventional Prospective Observational Study of Platelet Rich Fibrin as a Therapy Adjunctive in Patients with Medication-Related Osteonecrosis of the Jaw
- PMID: 35160132
- PMCID: PMC8837070
- DOI: 10.3390/jcm11030682
Non-Interventional Prospective Observational Study of Platelet Rich Fibrin as a Therapy Adjunctive in Patients with Medication-Related Osteonecrosis of the Jaw
Abstract
Background: Medication-related osteonecrosis (MRONJ) of the jaw is a severe and feared side effect of antiresorptive therapy in the oncological setting. With growing evidence that impaired angiogenesis may represent a key factor in pathogenesis, the aim of this study was to evaluate an autologous platelet concentrate as a possible additive in surgical therapy to optimize vascularization and, subsequently, resolution rates.
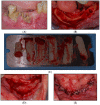
Material and methods: A non-interventional, prospective, multicenter study was conducted, and all patients with stage I-III MRONJ, undergoing antiresorptive therapy for an oncological indication, were included. The necrosis was treated surgically without (study arm A) or with (arm B) the addition of an autologous platelet concentrate (platelet-rich fibrin, PRF).
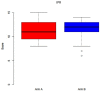
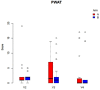
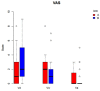
Results: After 5, 14, and 42 days postoperative, wound healing (primary outcome: mucosal integrity) as well as downstaging, pain perception, and oral health-related quality of life (secondary outcome) were assessed via clinical evaluation. Among the 52 patients included, primarily with MRONJ stage I and II, the use of PRF as an additive in surgical therapy did not display a significant advantage for wound healing (p = 0.302), downstaging (p = 0.9), pain reduction (p = 0.169), or quality of life (p = 0.9).
Summary: In conclusion, PRF as an adjunct did not significantly optimize wound healing. Further, no significant changes in terms of downstaging, pain sensation, and oral health-related quality of life were found.
Keywords: MRONJ; angiogenesis; platelet rich fibrin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Bamias A., Kastritis E., Bamia C., Moulopoulos L.A., Melakopoulos I., Bozas G., Koutsoukou V., Gika D., Anagnostopoulos A., Papadimitriou C., et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J. Clin. Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. - DOI - PubMed
-
- Walter C., Al-Nawas B., Grötz K.A., Thomas C., Thüroff J.W., Zinser V., Gamm H., Beck J., Wagner W. Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur. Urol. 2008;54:1066–1072. doi: 10.1016/j.eururo.2008.06.070. - DOI - PubMed
-
- Khan A.A., Morrison A., Hanley D.A., Felsenberg D., McCauley L.K., O’Ryan F., Reid I.R., Ruggiero S.L., Taguchi A., Tetradis S., et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015;30:3–23. doi: 10.1002/jbmr.2405. - DOI - PubMed
-
- Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O’Ryan F., American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteone-crosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

