Perioperative Management of Patients with Cardiac Implantable Electronic Devices and Utility of Magnet Application
- PMID: 35160149
- PMCID: PMC8836758
- DOI: 10.3390/jcm11030691
Perioperative Management of Patients with Cardiac Implantable Electronic Devices and Utility of Magnet Application
Abstract
With the demographic evolution of the population, patients undergoing surgery today are older and have an increasing number of sometimes complex comorbidities. Cardiac implantable electronic devices (CIED) are also getting more and more complex with very sophisticated programming algorithms. It may be generally assumed that magnet application reverts pacing to an asynchronous mode in pacemakers and disables tachycardia detection/therapy in internal cardioverter-defibrillators. However, depending on device type, manufacturer and model, the response to magnet application may differ substantially. For these reasons, perioperative management of CIED patients is getting more and more challenging. With this review article we provide an overview of optimal perioperative management of CIED patients with a detailed description of CIED response to magnet application depending on manufacturer and device-type, which may help in providing a safe perioperative management plan for the CIED patient.
Keywords: cardiac implantable electronic device; cardiac resynchronization therapy; implantable cardioverter defibrillator; magnet application; pacemaker; perioperative management.
Conflict of interest statement
A.A. is a consultant to Boston Scientific, Cairdac, Corvia, MicroportCRM, EPD Philips, Radcliffe Publisher. He received speaker fees from Boston Scientific, Medtronic, and Microport. He participates in clinical trials sponsored by Boston Scientific, Medtronic, EPD-Philips. He holds intellectual properties with Boston Scientific, Biosense Webster, and Microport CRM. T.O., M.L.C., A.D. and E.B. have no conflict of interest.
Figures

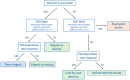
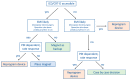
References
-
- Greenspon A.J., Patel J.D., Lau E., Ochoa J., Frisch D.R., Ho R.T., Pavri B.B., Kurtz S.M. 16-Year Trends in the Infection Burden for Pacemakers and Implantable Cardioverter-Defibrillators in the United States: 1993 to 2008. J. Am. Coll. Cardiol. 2011;58:1001–1006. doi: 10.1016/j.jacc.2011.04.033. - DOI - PubMed
-
- WHO Regional Office for Europe European Health Information Gateway. “Inpatient Surgical Procedures Per Year Per 100,000” European Health for All Explorer. [(accessed on 8 March 2021)]. Available online: https://gateway.euro.who.int/en/indicators/hfa_538-6030-inpatient-surgic...
Publication types
LinkOut - more resources
Full Text Sources

