Combination of CLEC4M rs868875 G-Carriership and ABO O Genotypes May Predict Faster Decay of FVIII Infused in Hemophilia A Patients
- PMID: 35160186
- PMCID: PMC8837058
- DOI: 10.3390/jcm11030733
Combination of CLEC4M rs868875 G-Carriership and ABO O Genotypes May Predict Faster Decay of FVIII Infused in Hemophilia A Patients
Abstract
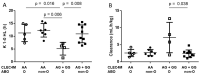
The C-type lectin CLEC4M binds and internalizes factor VIII (FVIII). Common CLEC4M variants have been associated with FVIII pharmacokinetic (PK) profiles in hemophilia A (HA) patients. The two-compartment PK analysis of plasma-derived (pd-) and full length recombinant FVIII concentrates was conducted in twenty-six patients (FVIII:C ≤ 2 IU/dL). F8, ABO blood-groups, and the CLEC4M rs868875A/G polymorphism were genotyped. CLEC4M genotype groups differed for the elimination rate constant K 1-0 (p < 0.001), half-life (K 1-0 HL), and the Beta rate constant. Patients treated with pd-FVIII also differed in the Alpha phase. In linear regression models, the contribution of the CLEC4M genotypes to FVIII PK parameters remained significant after correction for ABO, age, and VWF antigen levels at PK. Combined CLEC4M rs868875A/G and ABO genotypes displayed significant interaction (K 1-0, p = 0.014). Compared to other combined genotypes, the G-carriers/O genotypes showed half-reduced K 1-0 HL (p = 0.008), and faster FVIII clearance (mean 7.1 ± 2.2 mL/h/kg SE) than in the G-carriers/non-O (mean 2.4 ± 0.3 mL/h/kg SE), (p = 0.038). Comparison in HA patients recruited in several countries suggests that CLEC4M genotypes coherently influence infused FVIII half-life and clearance. Our analysis supports substantially faster FVIII decay associated with the rs868875 G-carrier/ABO O genotypes, which has potential implications for genetically tailored substitutive HA treatment.
Keywords: ABO; CLEC4M; CLEC4M SNPs; clearance; factor VIII; haemophilia A; half-life; pharmacokinetics.
Conflict of interest statement
M.M. reports personal fees from Kedrion, grants from Pfizer, personal fees from Novo Nordisk, personal fees from SOBI, personal fees from Bayer, personal fees from Bioverativ, personal fees from Octapharma, personal fees from CSL Behring, outside the submitted work. G.C. reports personal fees from Roche, personal fees from Bayer, personal fees from Shire, grants and personal fees from CSL Behring, grants and personal fees from Sobi, personal fees from Uniqure, grants from Pfizer, personal fees from Kedrion, personal fees from Novo Nordisk, personal fees from Werfen, outside the submitted work. F.B. reports grants from Bayer, during the conduct of the study; grants from Pfizer, outside the submitted work. The other authors state that they have no conflict of interest.
Figures
Similar articles
-
Current Understanding of Inherited Modifiers of FVIII Pharmacokinetic Variation.Pharmgenomics Pers Med. 2023 Mar 24;16:239-252. doi: 10.2147/PGPM.S383221. eCollection 2023. Pharmgenomics Pers Med. 2023. PMID: 36998673 Free PMC article. Review.
-
Common Genetic Variants in ABO and CLEC4M Modulate the Pharmacokinetics of Recombinant FVIII in Severe Hemophilia A Patients.Thromb Haemost. 2020 Oct;120(10):1395-1406. doi: 10.1055/s-0040-1714214. Epub 2020 Jul 29. Thromb Haemost. 2020. PMID: 32726853
-
Modulation of factor VIII pharmacokinetics by genetic components in factor VIII receptors.Haemophilia. 2023 Mar;29(2):479-487. doi: 10.1111/hae.14722. Epub 2022 Dec 19. Haemophilia. 2023. PMID: 36533781
-
Factor VIII pharmacokinetics associates with genetic modifiers of VWF and FVIII clearance in an adult hemophilia A population.J Thromb Haemost. 2021 Mar;19(3):654-663. doi: 10.1111/jth.15183. Epub 2020 Dec 31. J Thromb Haemost. 2021. PMID: 33219619
-
Biological mechanisms underlying inter-individual variation in factor VIII clearance in haemophilia.Haemophilia. 2020 Jul;26(4):575-583. doi: 10.1111/hae.14078. Epub 2020 Jun 28. Haemophilia. 2020. PMID: 32596930 Free PMC article. Review.
Cited by
-
Current Understanding of Inherited Modifiers of FVIII Pharmacokinetic Variation.Pharmgenomics Pers Med. 2023 Mar 24;16:239-252. doi: 10.2147/PGPM.S383221. eCollection 2023. Pharmgenomics Pers Med. 2023. PMID: 36998673 Free PMC article. Review.
-
Managing Relevant Clinical Conditions of Hemophilia A/B Patients.Hematol Rep. 2023 Jun 7;15(2):384-397. doi: 10.3390/hematolrep15020039. Hematol Rep. 2023. PMID: 37367088 Free PMC article.
-
The contribution of the sinusoidal endothelial cell receptors CLEC4M, stabilin-2, and SCARA5 to VWF-FVIII clearance in thrombosis and hemostasis.J Thromb Haemost. 2023 Aug;21(8):2007-2019. doi: 10.1016/j.jtha.2023.04.014. Epub 2023 Apr 19. J Thromb Haemost. 2023. PMID: 37085036 Free PMC article. Review.
References
-
- Vlot A.J., Mauser-Bunschoten E.P., Zarkova A.G., Haan E., Kruitwagen C.L., Sixma J.J., van den Berg H.M. The Half-Life of Infused Factor VIII Is Shorter in Hemophiliac Patients with Blood Group O than in Those with Blood Group A. Thromb. Haemost. 2000;83:65–69. - PubMed
-
- Morange P.E., Tregouet D.A., Frere C., Saut N., Pellegrina L., Alessi M.C., Visvikis S., Tiret L., Juhan-Vague I. Biological and Genetic Factors Influencing Plasma Factor VIII Levels in a Healthy Family Population: Results from the Stanislas Cohort. Br. J. Haematol. 2005;128:91–99. doi: 10.1111/j.1365-2141.2004.05275.x. - DOI - PubMed
-
- van Dijk K., van der Bom J.G., Lenting P.J., de Groot P.G., Mauser-Bunschoten E.P., Roosendaal G., Grobbee D.E., van den Berg H.M. Factor VIII Half-Life and Clinical Phenotype of Severe Hemophilia A. Haematologica. 2005;90:494–498. - PubMed
-
- Björkman S., Blanchette V.S., Fischer K., Oh M., Spotts G., Schroth P., Fritsch S., Patrone L., Ewenstein B.M., Advate Clinical Program Group et al. Comparative Pharmacokinetics of Plasma- and Albumin-Free Recombinant Factor VIII in Children and Adults: The Influence of Blood Sampling Schedule on Observed Age-Related Differences and Implications for Dose Tailoring. J. Thromb. Haemost. 2010;8:730–736. doi: 10.1111/j.1538-7836.2010.03757.x. - DOI - PMC - PubMed
-
- Martinelli N., Girelli D., Lunghi B., Pinotti M., Marchetti G., Malerba G., Pignatti P.F., Corrocher R., Olivieri O., Bernardi F. Polymorphisms at LDLR Locus May Be Associated with Coronary Artery Disease through Modulation of Coagulation Factor VIII Activity and Independently from Lipid Profile. Blood. 2010;116:5688–5697. doi: 10.1182/blood-2010-03-277079. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous