Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices
- PMID: 35160311
- PMCID: PMC8836477
- DOI: 10.3390/jcm11030860
Viscoelastic Hemostatic Assays: A Primer on Legacy and New Generation Devices
Abstract
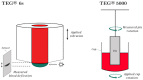
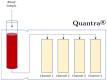
Viscoelastic hemostatic assay (VHAs) are whole blood point-of-care tests that have become an essential method for assaying hemostatic competence in liver transplantation, cardiac surgery, and most recently, trauma surgery involving hemorrhagic shock. It has taken more than three-quarters of a century of research and clinical application for this technology to become mainstream in these three clinical areas. Within the last decade, the cup and pin legacy devices, such as thromboelastography (TEG® 5000) and rotational thromboelastometry (ROTEM® delta), have been supplanted not only by cartridge systems (TEG® 6S and ROTEM® sigma), but also by more portable point-of-care bedside testing iterations of these legacy devices (e.g., Sonoclot®, Quantra®, and ClotPro®). Here, the legacy and new generation VHAs are compared on the basis of their unique hemostatic parameters that define contributions of coagulation factors, fibrinogen/fibrin, platelets, and clot lysis as related to the lifespan of a clot. In conclusion, we offer a brief discussion on the meteoric adoption of VHAs across the medical and surgical specialties to address COVID-19-associated coagulopathy.
Keywords: COVID-19; coagulopathy; fibrinogen; hemorrhage; heparin; personalized medicine; rotational thromboelastometry; thromboelastography; thrombosis.
Conflict of interest statement
O.V. is a consultant for Haemonetics Corporation (Boston, MA, USA), served on the Clinical Care Improvement Steering Committee for Diagnostica Stago, Inc., and received honoraria for consultancy outside the submitted work. E.E.M., H.B.M., M.D.N. and M.M.W. have received research grants from Haemonetics Corporation outside the submitted work. M.D.N. has received an honorarium from Haemonetics Corporation for speaking engagements, as well as research support from Janssen Pharmaceuticals (Beerse, Belgium) and Noveome Biotherapeutics (Pittsburgh, PA, USA) outside the submitted work. He has served as a consultant to Janssen and CSL Behring (King of Prussia, PA, USA) and serves on the Scientific Advisory Board of Haima Therapeutics (Cleveland, OH, USA). M.M.W. has received honoraria from Alexion Pharmaceuticals (Boston, MA, USA). D.F. has received study funding, honoraria for consultancy, and shows board activity from Astra Zeneca, AOP orphan, Baxter, Bayer, B. Braun Medical, Biotest, CSL Behring, Delta Select, Dade Behring, Edwards, Fresenius, Glaxo, Haemoscope, Hemogem, Lilly, LFB, Mitsubishi Pharma, Novo Nordisk, Octapharma, Pfizer, and Tem Innovations outside the submitted work.
Figures

References
-
- Kang Y.G., Martin D.J., Marquez J., Lewis J.H., Bontempo F.A., Shaw B.W., Jr., Starzl T.E., Winter P.M. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth. Analg. 1985;64:888–896. doi: 10.1213/00000539-198509000-00008. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

