Methylene Blue Dye as Photosensitizer for Scavenger-Less Water Photo Splitting: New Insight in Green Hydrogen Technology
- PMID: 35160513
- PMCID: PMC8839752
- DOI: 10.3390/polym14030523
Methylene Blue Dye as Photosensitizer for Scavenger-Less Water Photo Splitting: New Insight in Green Hydrogen Technology
Abstract
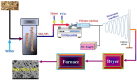
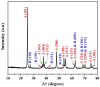
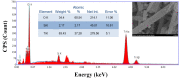
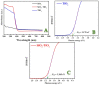
In this study, hydrogen generation was performed by utilizing methylene blue dye as visible-light photosensitizer while the used catalyst is working as a transfer bridge for the electrons to H+/H2 reaction. Silica NPs-incorporated TiO2 nanofibers, which have a more significant band gap and longer electrons lifetime compared to pristine TiO2, were used as a catalyst. The nanofibers were prepared by electrospinning of amorphous SiO2 NPs/titanium isopropoxide/poly (vinyl acetate)/N, N-dimethylformamide colloid. Physicochemical characterizations confirmed the preparation of well morphology SiO2-TiO2 nanofibers with a bandgap energy of 3.265 eV. Under visible light radiation, hydrogen and oxygen were obtained in good stoichiometric rates (9.5 and 4.7 mL/min/gcat, respectively) without any considerable change in the dye concentration, which proves the successful exploitation of the dye as a photosensitizer. Under UV irradiation, SiO2 NPs incorporation distinctly enhanced the dye photodegradation, as around 91 and 94% removal efficiency were obtained from TiO2 nanofibers containing 4 and 6 wt% of the used dopant, respectively, within 60 min.
Keywords: electrospinning; hydrogen; photosensitizer; silica nanoparticles; water photo splitting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Photocatalytic performance of electrospun CNT/TiO2 nanofibers in a simulated air purifier under visible light irradiation.Environ Sci Pollut Res Int. 2016 Nov;23(21):21395-21406. doi: 10.1007/s11356-016-7348-z. Epub 2016 Aug 9. Environ Sci Pollut Res Int. 2016. PMID: 27502566
-
Preparation of TiO2/WO3/C/N Composite Nanofibers by Electrospinning Using Precursors Soluble in Water and Their Photocatalytic Activity in Visible Light.Nanomaterials (Basel). 2021 Feb 1;11(2):351. doi: 10.3390/nano11020351. Nanomaterials (Basel). 2021. PMID: 33535415 Free PMC article.
-
CdTiO3-NPs incorporated TiO2 nanostructure photocatalyst for scavenger-free water splitting under visible radiation.PLoS One. 2022 Oct 18;17(10):e0276097. doi: 10.1371/journal.pone.0276097. eCollection 2022. PLoS One. 2022. PMID: 36256606 Free PMC article.
-
Hydrothermal-Assisted Synthesis of Copper Nanoparticles-Decorated Titania Nanofibers for Methylene Blue Photodegradation and Catalyst for Sodium Borohydride Dehydrogenation.Polymers (Basel). 2022 Nov 28;14(23):5180. doi: 10.3390/polym14235180. Polymers (Basel). 2022. PMID: 36501572 Free PMC article.
-
Photosensitization of TiO2 nanofibers by Ag2S with the synergistic effect of excess surface Ti3+ states for enhanced photocatalytic activity under simulated sunlight.Sci Rep. 2017 Mar 21;7(1):255. doi: 10.1038/s41598-017-00366-7. Sci Rep. 2017. PMID: 28325907 Free PMC article.
Cited by
-
A New Insight into the Mechanisms Underlying the Discoloration, Sorption, and Photodegradation of Methylene Blue Solutions with and without BNOx Nanocatalysts.Materials (Basel). 2022 Nov 17;15(22):8169. doi: 10.3390/ma15228169. Materials (Basel). 2022. PMID: 36431653 Free PMC article.
-
Extraction of Novel Effective Nanocomposite Photocatalyst from Corn Stalk for Water Photo Splitting under Visible Light Radiation.Polymers (Basel). 2022 Dec 30;15(1):185. doi: 10.3390/polym15010185. Polymers (Basel). 2022. PMID: 36616535 Free PMC article.
-
Impact of Polymers on Magnesium-Based Hydrogen Storage Systems.Polymers (Basel). 2022 Jun 27;14(13):2608. doi: 10.3390/polym14132608. Polymers (Basel). 2022. PMID: 35808653 Free PMC article. Review.
-
Photocatalytic activity in graded off-valent cations substituted NaNbO3.Heliyon. 2024 Apr 2;10(7):e29121. doi: 10.1016/j.heliyon.2024.e29121. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38617944 Free PMC article.
-
TiO2 NPs-immobilized silica granules: New insight for nano catalyst fixation for hydrogen generation and sustained wastewater treatment.PLoS One. 2023 Jun 21;18(6):e0287424. doi: 10.1371/journal.pone.0287424. eCollection 2023. PLoS One. 2023. PMID: 37343028 Free PMC article.
References
-
- Morrison S.R. The Chemical Physics of Surfaces. Springer Science & Business Media; New York, NY, USA: 2013.
-
- Kampouri S., Stylianou K.C. Dual-functional photocatalysis for simultaneous hydrogen production and oxidation of organic substances. ACS Catal. 2019;9:4247–4270. doi: 10.1021/acscatal.9b00332. - DOI
-
- Linkous C.A., Huang C., Fowler J.R. UV photochemical oxidation of aqueous sodium sulfide to produce hydrogen and sulfur. J. Photochem. Photobiol. A. 2004;168:153–160. doi: 10.1016/j.jphotochem.2004.03.028. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

