Solubility and Dissolution Enhancement of Dexibuprofen with Hydroxypropylbetacyclodextrin (HPβCD) and Poloxamers (188/407) Inclusion Complexes: Preparation and In Vitro Characterization
- PMID: 35160569
- PMCID: PMC8838044
- DOI: 10.3390/polym14030579
Solubility and Dissolution Enhancement of Dexibuprofen with Hydroxypropylbetacyclodextrin (HPβCD) and Poloxamers (188/407) Inclusion Complexes: Preparation and In Vitro Characterization
Abstract
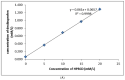
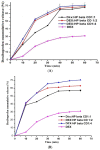
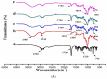
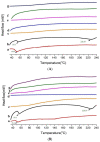
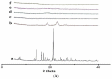
The objective of this study was to improve the dissolution and solubility of dexibuprofen (DEX) using hydroxypropyl beta cyclodextrin (HPβCD) inclusion complexes and also to evaluate the effect of presence of hydrophilic polymers on solubilization efficiency of HPβCD. Three different methods (physical trituration, kneading and solvent evaporation) were used to prepare binary inclusion complexes at various drug-to-cyclodextrin weight ratios. An increase in solubility and drug release was observed with the kneading (KN) method at a DEX/HPβCD (1:4) weight ratio. The addition of hydrophilic polymers poloxamer-188 (PXM-188) and poloxamer-407 (PXM-407) at 2.5, 5.0, 10.0 and 20% w/w enhanced the complexation efficiency and solubility of DEX/HPβCD significantly. Fourier-transform infrared (FTIR) analysis revealed that DEX was successfully incorporated into the cyclodextrin cavity. Differential scanning calorimetry (DSC) and X-ray diffractometry (XRD) revealed less crystallinity of the drug and its entrapment in the cyclodextrin molecular cage. The addition of PXM-188 or PXM-407 reduced the strength of the DEX endothermic peak. With the addition of hydrophilic polymers, sharp and intense peaks of DEX disappeared. Finally, it was concluded that PXM-188 at a weight ratio of 10.0% w/w was the best candidate for improving solubility, stability and release rate of DEX.
Keywords: HPβCD; PXM; dexibuprofen; inclusion complex.
Conflict of interest statement
There is no conflict of interest for any of the listed authors.
Figures






Similar articles
-
Effect of Hydrophilic Polymers on Complexation Efficiency of Cyclodextrins in Enhancing Solubility and Release of Diflunisal.Polymers (Basel). 2020 Jul 15;12(7):1564. doi: 10.3390/polym12071564. Polymers (Basel). 2020. PMID: 32679660 Free PMC article.
-
Effect of hydrophilic polymers on the solubility and dissolution enhancement of rivaroxaban/beta-cyclodextrin inclusion complexes.Heliyon. 2023 Sep 1;9(9):e19658. doi: 10.1016/j.heliyon.2023.e19658. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809727 Free PMC article.
-
Influence of hydrophilic polymers on celecoxib complexation with hydroxypropyl β-cyclodextrin.AAPS PharmSciTech. 2006 Sep;7(3):E184-E189. doi: 10.1208/pt070379. Epub 2017 Mar 8. AAPS PharmSciTech. 2006. PMID: 28290014
-
Effect of hydrophilic polymers on isradipine complexation with hydroxypropyl β-cyclodextrin.Drug Dev Ind Pharm. 2013 Jul;39(7):970-7. doi: 10.3109/03639045.2012.686508. Epub 2012 May 21. Drug Dev Ind Pharm. 2013. PMID: 22612875
-
Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir.J Pharm Biomed Anal. 2008 Jul 15;47(3):535-40. doi: 10.1016/j.jpba.2008.02.006. Epub 2008 Feb 15. J Pharm Biomed Anal. 2008. PMID: 18367363 Review.
Cited by
-
QbD Assisted Systematic Review for Optimizing the Selection of PVP as a Ternary Substance in Enhancing the Complexation Efficiency of Cyclodextrins: a Pilot Study.AAPS PharmSciTech. 2024 Jun 11;25(5):134. doi: 10.1208/s12249-024-02845-3. AAPS PharmSciTech. 2024. PMID: 38862663
-
Microwave-Assisted Formation of Ternary Inclusion Complex of Pterostilbene.Pharmaceuticals (Basel). 2023 Nov 22;16(12):1641. doi: 10.3390/ph16121641. Pharmaceuticals (Basel). 2023. PMID: 38139768 Free PMC article.
-
Continuous Manufacturing of Solvent-Free Cyclodextrin Inclusion Complexes for Enhanced Drug Solubility via Hot-Melt Extrusion: A Quality by Design Approach.Pharmaceutics. 2023 Aug 25;15(9):2203. doi: 10.3390/pharmaceutics15092203. Pharmaceutics. 2023. PMID: 37765172 Free PMC article.
-
Enhanced Solubility and Biological Activity of Dexibuprofen-Loaded Silica-Based Ternary Solid Dispersions.Pharmaceutics. 2023 Jan 24;15(2):399. doi: 10.3390/pharmaceutics15020399. Pharmaceutics. 2023. PMID: 36839721 Free PMC article.
-
Preparation, Characterization, and Evaluation of Physcion Nanoparticles for Enhanced Oral Bioavailability: An Attempt to Improve Its Antioxidant and Anticancer Potential.ACS Omega. 2023 Sep 11;8(37):33955-33965. doi: 10.1021/acsomega.3c04821. eCollection 2023 Sep 19. ACS Omega. 2023. PMID: 37744808 Free PMC article.
References
-
- Krishnaiah Y.S. Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J. Bioequiv. Bioavailab. 2010;2:28–36. doi: 10.4172/jbb.1000027. - DOI
-
- Kumar A., Sahoo S.K., Padhee K., Kochar P., Satapathy A., Pathak N. Review on solubility enhancement techniques for hydrophobic drugs. Pharm. Glob. 2011;3:1–7.
-
- Crupi V., Majolino D., Mele A., Rossi B., Trotta F., Venuti V. Modelling the interplay between covalent and physical interactions in cyclodextrin-based hydrogel: Effect of water confinement. Soft Matter. 2013;9:6457–6464. doi: 10.1039/c3sm50827g. - DOI
LinkOut - more resources
Full Text Sources

