Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes
- PMID: 35160720
- PMCID: PMC8836503
- DOI: 10.3390/ma15030775
Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes
Abstract
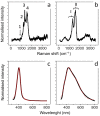
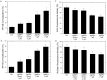
The use of silver nanoparticles (Ag NPs) in the biomedical field deserves a mindful analysis of the possible inflammatory response which could limit their use in the clinic. Despite the anti-cancer properties of Ag NPs having been widely demonstrated, there are still few studies concerning their involvement in the activation of specific inflammatory pathways. The inflammatory outcome depends on the synthetic route used in the NPs production, in which toxic reagents are employed. In this work, we compared two types of Ag NPs, obtained by two different chemical routes: conventional synthesis using sodium citrate and a green protocol based on leaf extracts as a source of reduction and capping agents. A careful physicochemical characterization was carried out showing spherical and stable Ag NPs with an average size between 20 nm and 35 nm for conventional and green Ag NPs respectively. Then, we evaluated their ability to induce the activation of inflammation in Human Leukemic Monocytes (THP-1) differentiated into M0 macrophages using 1 µM and 2 µM NPs concentrations (corresponded to 0.1 µg/mL and 0.2 µg/mL respectively) and two-time points (24 h and 48 h). Our results showed a clear difference in Nuclear Factor κB (NF-κb) activation, Interleukins 6-8 (IL-6, IL-8) secretion, Tumor Necrosis Factor-α (TNF-α) and Cyclooxygenase-2 (COX-2) expression exerted by the two kinds of Ag NPs. Green Ag NPs were definitely tolerated by macrophages compared to conventional Ag NPs which induced the activation of all the factors mentioned above. Subsequently, the exposure of breast cancer cell line (MCF-7) to the green Ag NPs showed that they exhibited antitumor activity like the conventional ones, but surprisingly, using the MCF-10A line (not tumoral breast cells) the green Ag NPs did not cause a significant decrease in cell viability.
Keywords: green route; inflammation response; physico-chemical properties; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Rai M., Birla S., Ingle A.P., Gupta I., Gade A., Abd-Elsalam K., Marcato P.D., Durán N. Nanosilver: An inorganic nanoparticle with myriad potential applications. Nanotechnol. Rev. 2014;3:281–309. doi: 10.1515/ntrev-2014-0001. - DOI
-
- De Matteis V., Rizzello L., Di Bello M.P., Rinaldi R. One-step synthesis, toxicity assessment and degradation in tumoral pH environment of SiO2@Ag core/shell nanoparticles. J. Nanopart. Res. 2017;19:14. doi: 10.1007/s11051-017-3870-2. - DOI
-
- Amendola V., Bakr O.M., Stellacci F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics. 2010;5:85–97. doi: 10.1007/s11468-009-9120-4. - DOI
-
- Govindappa M., Tejashree S., Thanuja V., Hemashekhar B., Srinivas C., Nasif O., Pugazhendhi A., Raghavendra V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Sci. Technol. 2020;61:102289. doi: 10.1016/j.jddst.2020.102289. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

