A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction
- PMID: 35160764
- PMCID: PMC8837119
- DOI: 10.3390/ma15030821
A Facile Synthesis of Noble-Metal-Free Catalyst Based on Nitrogen Doped Graphene Oxide for Oxygen Reduction Reaction
Abstract
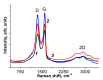
A simple method for the mechanochemical synthesis of an effective metal-free electrocatalyst for the oxygen reduction reaction was demonstrated. A nitrogen-doped carbon material was obtained by grinding a mixture of graphene oxide and melamine in a planetary ball mill. The resulting material was characterized by XPS, EPR, and Raman and IR spectroscopy. The nitrogen concentration on the N-bmGO surface was 5.5 at.%. The nitrogen-enriched graphene material (NbmGO has half-wave potential of -0.175/-0.09 V and was shown to possess high activity as an electrocatalyst for oxygen reduction reaction. The electrocatalytic activity of NbmGO can be associated with a high concentration of active sites for the adsorption of oxygen molecules on its surface. The high current retention (93% for 12 h) after continuous polarization demonstrates the excellent long-term stability of NbmGO.
Keywords: N-doped; ball-milling; graphene oxide; melamine; noble-metal-free catalysts; oxygen reduction reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Majlan E.H., Rohendi D., Daud W.R.W., Husaini T., Haque M.A. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018;89:117–134. doi: 10.1016/j.rser.2018.03.007. - DOI
-
- Shao Q., Li F.M., Chen Y., Huang X.Q. The advanced designs of high-performance platinum-based electrocatalysts: Recent progresses and challenges. Adv. Mater. Interfaces. 2018;5:1800486. doi: 10.1002/admi.201800486. - DOI
-
- Gasteiger H.A., Kocha S.S., Sompalli B., Wagner F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005;56:9–35. doi: 10.1016/j.apcatb.2004.06.021. - DOI
-
- Nørskov J.K., Rossmeisl J., Logadottir A., Lindqvist L., Kitchin J.R., Bligaard T., Jónsson H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004;108:17886–17892. doi: 10.1021/jp047349j. - DOI
LinkOut - more resources
Full Text Sources

