Mineralization in a Critical Size Bone-Gap in Sheep Tibia Improved by a Chitosan-Calcium Phosphate-Based Composite as Compared to Predicate Device
- PMID: 35160784
- PMCID: PMC8836995
- DOI: 10.3390/ma15030838
Mineralization in a Critical Size Bone-Gap in Sheep Tibia Improved by a Chitosan-Calcium Phosphate-Based Composite as Compared to Predicate Device
Abstract
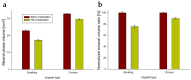
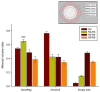
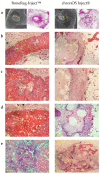
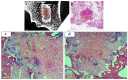
Deacetylated chitin derivatives have been widely studied for tissue engineering purposes. This study aimed to compare the efficacy of an injectable product containing a 50% deacetylated chitin derivative (BoneReg-Inject™) and an existing product (chronOS Inject®) serving as a predicate device. A sheep model with a critical size drill hole in the tibial plateau was used. Holes of 8 mm diameter and 30 mm length were drilled bilaterally into the proximal area of the tibia and BoneReg-Inject™ or chronOS Inject® were injected into the right leg holes. Comparison of resorption and bone formation in vivo was made by X-ray micro-CT and histological evaluation after a live phase of 12 weeks. Long-term effects of BoneReg-Inject™ were studied using a 13-month live period. Significant differences were observed in (1) amount of new bone within implant (p < 0.001), higher in BoneReg-InjectTM, (2) signs of cartilage tissue (p = 0.003), more pronounced in BoneReg-InjectTM, and (3) signs of fibrous tissue (p < 0.001), less pronounced in BoneReg-InjectTM. Mineral content at 13 months postoperative was significantly higher than at 12 weeks (p < 0.001 and p < 0.05, for implant core and rim, respectively). The data demonstrate the potential of deacetylated chitin derivatives to stimulate bone formation.
Keywords: X-ray micro CT; bone defects; bone formation; bone implant; chitosan; degree of deacetylation; histology; sheep tibia.
Conflict of interest statement
Jóhannes Gíslason, Jón M. Einarsson and Chuen-How Ng are employees of Genis hf.
Figures



References
-
- Dennis S.C., Berkland C.J., Bonewald L.F., Detamore M.S. Endochondral ossification for enhancing bone regeneration: Converging native extracellular matrix biomaterials and developmental engineering in vivo. Tissue Eng. Part B Rev. 2015;21:247–266. doi: 10.1089/ten.teb.2014.0419. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

