Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation
- PMID: 35160982
- PMCID: PMC8838330
- DOI: 10.3390/ma15031037
Carbon Nanomaterials (CNMs) and Enzymes: From Nanozymes to CNM-Enzyme Conjugates and Biodegradation
Abstract
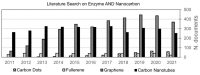
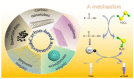
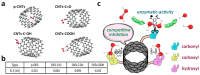
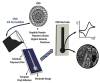
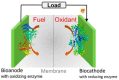
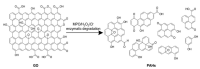
Carbon nanomaterials (CNMs) and enzymes differ significantly in terms of their physico-chemical properties-their handling and characterization require very different specialized skills. Therefore, their combination is not trivial. Numerous studies exist at the interface between these two components-especially in the area of sensing-but also involving biofuel cells, biocatalysis, and even biomedical applications including innovative therapeutic approaches and theranostics. Finally, enzymes that are capable of biodegrading CNMs have been identified, and they may play an important role in controlling the environmental fate of these structures after their use. CNMs' widespread use has created more and more opportunities for their entry into the environment, and thus it becomes increasingly important to understand how to biodegrade them. In this concise review, we will cover the progress made in the last five years on this exciting topic, focusing on the applications, and concluding with future perspectives on research combining carbon nanomaterials and enzymes.
Keywords: carbon nano-onions; carbon nanodots; carbon nanomaterials; carbon nanotubes; enzymes; fuel cells; graphene; medicine; nanozymes; sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chaudhary K., Kumar K., Venkatesu P., Masram D.T. Protein immobilization on graphene oxide or reduced graphene oxide surface and their applications: Influence over activity, structural and thermal stability of protein. Adv. Colloid Interface Sci. 2021;289:102367. doi: 10.1016/j.cis.2021.102367. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

