Synthesis of Novel Nano-Sulfonamide Metal-Based Corrosion Inhibitor Surfactants
- PMID: 35161090
- PMCID: PMC8838271
- DOI: 10.3390/ma15031146
Synthesis of Novel Nano-Sulfonamide Metal-Based Corrosion Inhibitor Surfactants
Abstract
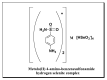
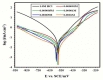
The synthesis of novel corrosion inhibitors and biocide metal complex nanoparticle surfactants was achieved through the reaction of sulfonamide with selenious acid to produce a quaternary ammonium salt. Platinum and cobalt surfactants were then formed by complexing the first products with platinum (II) or cobalt (II) ions. The surface properties of these surfactants were then investigated, and the free energy of form micelles (ΔGomic) and adsorption (ΔGoads) was determined. The obtained cationic compounds were evaluated as corrosion inhibitors for carbon steel dissolution in 1N HCl medium. The results of gravimetric and electrochemical measurements showed that the obtained inhibitors were excellent corrosion inhibitors. The anti-sulfate-reducing bacteria activity known to cause corrosion of oil pipes was obtained by the inhibition zone diameter method for the prepared compounds, which were measured against sulfate-reducing bacteria. FTIR spectra, elemental analysis, H1 NMR spectrum, and 13C labeling were performed to ensure the purity of the prepared compounds.
Keywords: antitumor activity; cationic surfactant; corrosion; critical micelle concentration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis and Biocidal Activity of Some Naphthalene-Based Cationic Surfactants.J Surfactants Deterg. 2012 Mar;15(2):223-234. doi: 10.1007/s11743-011-1286-z. Epub 2011 Jul 30. J Surfactants Deterg. 2012. PMID: 22389580 Free PMC article.
-
Surface and antitumor activity of some novel metal-based cationic surfactants.J Cancer Res Ther. 2007 Oct-Dec;3(4):198-206. doi: 10.4103/0973-1482.38994. J Cancer Res Ther. 2007. PMID: 18270394
-
Two novel triazine-based quaternary ammonium salt Gemini surfactants as potential corrosion inhibitors for carbon steel in a sulfate-reducing bacteria solution: Experimental and theoretical studies.Heliyon. 2024 Nov 20;10(23):e40385. doi: 10.1016/j.heliyon.2024.e40385. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39660201 Free PMC article.
-
Functional chitosan surfactants for corrosion inhibition in petroleum industry.Int J Biol Macromol. 2024 Oct;278(Pt 2):134704. doi: 10.1016/j.ijbiomac.2024.134704. Epub 2024 Aug 13. Int J Biol Macromol. 2024. PMID: 39147343 Review.
-
Surfactants as biodegradable sustainable inhibitors for corrosion control in diverse media and conditions: A comprehensive review.Sci Total Environ. 2024 Jan 15;908:168407. doi: 10.1016/j.scitotenv.2023.168407. Epub 2023 Nov 7. Sci Total Environ. 2024. PMID: 37939963 Review.
Cited by
-
Effect of Quaternary Ammonium Salts and 1,2,4-Triazole Derivatives on Hydrogen Absorption by Mild Steel in Hydrochloric Acid Solution.Materials (Basel). 2022 Oct 8;15(19):6989. doi: 10.3390/ma15196989. Materials (Basel). 2022. PMID: 36234330 Free PMC article.
-
Gallic acid based green corrosion inhibitor for mild steel in 1 M HCl electrochemical and microbial assessment with theoretical validation.Sci Rep. 2025 Apr 30;15(1):15156. doi: 10.1038/s41598-025-97647-3. Sci Rep. 2025. PMID: 40307297 Free PMC article.
-
Study on the Resistance of Concrete to High-Concentration Sulfate Attack: A Case Study in Jinyan Bridge.Materials (Basel). 2024 Jul 9;17(14):3388. doi: 10.3390/ma17143388. Materials (Basel). 2024. PMID: 39063678 Free PMC article.
-
Utilizing Some Indole Derivatives to Control Mild Steel Corrosion in Acidic Environments: Electrochemical and Theoretical Methods.Molecules. 2025 Mar 10;30(6):1235. doi: 10.3390/molecules30061235. Molecules. 2025. PMID: 40142011 Free PMC article.
-
An examination of the effectiveness of the expired drug isoprinosine in preventing aluminum corrosion in alkaline solutions using both computational and experimental techniques.RSC Adv. 2024 Apr 8;14(16):11244-11257. doi: 10.1039/d4ra00158c. eCollection 2024 Apr 3. RSC Adv. 2024. PMID: 38590354 Free PMC article.
References
-
- Verma C., Haque J., Quraishi M.A., Ebenso E.E. Aqueous phase environmental friendly organic corrosion inhibitors derived from one step multicomponent reactions: A review. J. Mol. Liq. 2018;275:18–40. doi: 10.1016/j.molliq.2018.11.040. - DOI
-
- Verma C., Ebenso E.E., Quraishi M. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017;233:403–414. doi: 10.1016/j.molliq.2017.02.111. - DOI
-
- Popova A. Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros. Sci. 2007;49:2144–2158. doi: 10.1016/j.corsci.2006.10.020. - DOI
-
- Ahamad I., Quraishi M.A. Bis-(benzimidazol-2-yl) disulphide: An efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros. Sci. 2009;51:2006–2013. doi: 10.1016/j.corsci.2009.05.026. - DOI
-
- Obot I.B., Obi-Egbedi N.O. Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: Experimental and theoretical investigation. Corros. Sci. 2010;52:198–204. doi: 10.1016/j.corsci.2009.09.002. - DOI
LinkOut - more resources
Full Text Sources

