Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents
- PMID: 35161102
- PMCID: PMC8837949
- DOI: 10.3390/ma15031158
Theoretical Mechanism on the Cellulose Regeneration from a Cellulose/EmimOAc Mixture in Anti-Solvents
Abstract
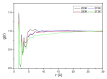
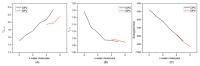
The experiments on cellulose dissolution/regeneration have made some achievements to some extent, but the mechanism of cellulose regeneration in ionic liquids (ILs) and anti-solvent mixtures remains elusive. In this work, the cellulose regeneration mechanism in different anti-solvents, and at different temperatures and concentrations, has been studied with molecular dynamics (MD) simulations. The IL considered is 1-ethyl-3-methylimidazolium acetate (EmimOAc). In addition, to investigate the microcosmic effects of ILs and anti-solvents, EmimOAc-nH2O (n = 0-6) clusters have been optimized by Density Functional Theory (DFT) calculations. It can be found that water is beneficial to the regeneration of cellulose due to its strong polarity. The interactions between ILs and cellulose will become strong with the increase in temperature. The H-bonds of cellulose chains would increase with the rising concentrations of anti-solvents. The interaction energies between cellulose and the anions of ILs are stronger than that of cations. Furthermore, the anti-solvents possess a strong affinity for ILs, cation-anion pairs are dissociated to form H-bonds with anti-solvents, and the H-bonds between cellulose and ILs are destroyed to promote cellulose regeneration.
Keywords: cellulose; ionic liquids; regeneration; theoretical calculations.
Conflict of interest statement
The authors have declared that there are no competing interests in their work.
Figures






Similar articles
-
Cellulose Regeneration in Imidazolium-Based Ionic Liquids and Antisolvent Mixtures: A Density Functional Theory Study.ACS Omega. 2022 Nov 7;7(46):42170-42180. doi: 10.1021/acsomega.2c04915. eCollection 2022 Nov 22. ACS Omega. 2022. PMID: 36440146 Free PMC article.
-
Cellulose Crystal Dissolution in Imidazolium-Based Ionic Liquids: A Theoretical Study.J Phys Chem B. 2018 Jan 11;122(1):258-266. doi: 10.1021/acs.jpcb.7b09525. Epub 2017 Dec 21. J Phys Chem B. 2018. PMID: 29264920
-
Understanding the interactions of cellulose with ionic liquids: a molecular dynamics study.J Phys Chem B. 2010 Apr 1;114(12):4293-301. doi: 10.1021/jp9117437. J Phys Chem B. 2010. PMID: 20218725
-
Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives.Appl Microbiol Biotechnol. 2017 Jan;101(2):521-532. doi: 10.1007/s00253-016-8057-8. Epub 2016 Dec 24. Appl Microbiol Biotechnol. 2017. PMID: 28012046 Review.
-
Towards a molecular understanding of cellulose dissolution in ionic liquids: anion/cation effect, synergistic mechanism and physicochemical aspects.Chem Sci. 2018 Mar 26;9(17):4027-4043. doi: 10.1039/c7sc05392d. eCollection 2018 May 7. Chem Sci. 2018. PMID: 29780532 Free PMC article. Review.
Cited by
-
Cellulose Membranes: Synthesis and Applications for Water and Gas Separation and Purification.Membranes (Basel). 2024 Jun 30;14(7):148. doi: 10.3390/membranes14070148. Membranes (Basel). 2024. PMID: 39057656 Free PMC article. Review.
-
Cellulose Regeneration in Imidazolium-Based Ionic Liquids and Antisolvent Mixtures: A Density Functional Theory Study.ACS Omega. 2022 Nov 7;7(46):42170-42180. doi: 10.1021/acsomega.2c04915. eCollection 2022 Nov 22. ACS Omega. 2022. PMID: 36440146 Free PMC article.
References
-
- Focher B., Palma M.T., Canetti M., Torri G., Cosentino C., Gastaldi G. Structural differences between non-wood plant celluloses: Evidence from solid state NMR, vibrational spectroscopy and X-ray diffractometry. Ind. Crops Prod. 2001;13:193–208. doi: 10.1016/S0926-6690(00)00077-7. - DOI
-
- Muhammad N., Man Z., Bustam M. Ionic liquid—A future solvent for the enhanced uses of wood biomass. Eur. J. Wood Wood Prod. 2011;70:125–133. doi: 10.1007/s00107-011-0526-2. - DOI
-
- O’Sullivan A.C. Cellulose: The structure slowly unravels. Cellulose. 1997;4:173–207. doi: 10.1023/A:1018431705579. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

