A New Jasmine Virus C Isolate Identified by Nanopore Sequencing Is Associated to Yellow Mosaic Symptoms of Jasminum officinale in Italy
- PMID: 35161290
- PMCID: PMC8839810
- DOI: 10.3390/plants11030309
A New Jasmine Virus C Isolate Identified by Nanopore Sequencing Is Associated to Yellow Mosaic Symptoms of Jasminum officinale in Italy
Abstract
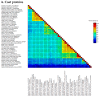
Some plants of Jasminum officinale were selected in a nursery for investigation of sanitary status of candidate mother plants before vegetative propagation. The presence of yellow spots and leaf discoloration symptoms pushed for a generic diagnosis through deep sequencing to discover systemic pathogens. Either dsRNA or total RNA were extracted and used in nanopore and Illumina platform for cDNA-PCR, direct RNA and total RNA rRNA-depleted sequencing. A few single reads obtained by nanopore technology or assembled contigs gave unequivocal annotation for the only presence of a jasmine virus C (JaVC, a putative member of genus Carlavirus) isolate. The full-length genome of this isolate was reconstructed, spanning 8490 nucleotides (nt). This isolate shared 90.9% similarity with coat protein sequences and 84% with the entire ORF1 polyprotein, with the other two available JaVC full genomes, isolated from infections in J. sambac in Taiwan and China. The overall nucleotide identity shared by the newly discovered Italian isolate with the Chinese JaVC full genomes was 76.14% (Taiwan) and 75.60% (Fujian). The application of quick nanopore sequencing for virus discovery was assessed. The identification of the virus in a new ornamental host species, largely used in gardening, creates a concern for the potential virus spread and need of testing for production of clean vegetative material.
Keywords: Carlavirus; high-throughput sequencing; jasmine; virus; virus detection; yellow mosaic.
Conflict of interest statement
The authors declare no conflict of interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Koenig R. Recently Discovered Virus or Viruslike Diseases of Ornamentals and Their Epidemiological Significance. Acta Hortic. 1985;164:21–32. doi: 10.17660/ActaHortic.1985.164.1. - DOI
-
- Gera A., Zeidan M. New and emerging virus diseases in ornamental crops. proc. xith is on virus diseases in Ornamentals. Acta Hortic. 2006;722:175–180. doi: 10.17660/ActaHortic.2006.722.21. - DOI
-
- Kreuze J.F., Perez A., Untiveros M., Quispe D., Fuentes S., Barker I., Simon R. Complete Viral Genome Sequence and Discovery of Novel Viruses by Deep Sequencing of Small RNAs: A Generic Method for Diagnosis, Discovery and Sequencing of Viruses. Virology. 2009;388:1–7. doi: 10.1016/j.virol.2009.03.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

