Genome-Wide Association Reveals Trait Loci for Seed Glucosinolate Accumulation in Indian Mustard (Brassica juncea L.)
- PMID: 35161346
- PMCID: PMC8838242
- DOI: 10.3390/plants11030364
Genome-Wide Association Reveals Trait Loci for Seed Glucosinolate Accumulation in Indian Mustard (Brassica juncea L.)
Abstract
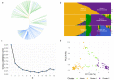
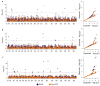
Glucosinolates (GSLs) are sulphur- and nitrogen-containing secondary metabolites implicated in the fitness of Brassicaceae and appreciated for their pungency and health-conferring properties. In Indian mustard (Brassica juncea L.), GSL content and composition are seed-quality-determining traits affecting its economic value. Depending on the end use, i.e., condiment or oil, different GSL levels constitute breeding targets. The genetic control of GSL accumulation in Indian mustard, however, is poorly understood, and current knowledge of GSL biosynthesis and regulation is largely based on Arabidopsis thaliana. A genome-wide association study was carried out to dissect the genetic architecture of total GSL content and the content of two major GSLs, sinigrin and gluconapin, in a diverse panel of 158 Indian mustard lines, which broadly grouped into a South Asia cluster and outside-South-Asia cluster. Using 14,125 single-nucleotide polymorphisms (SNPs) as genotyping input, seven distinct significant associations were discovered for total GSL content, eight associations for sinigrin content and 19 for gluconapin. Close homologues of known GSL structural and regulatory genes were identified as candidate genes in proximity to peak SNPs. Our results provide a comprehensive map of the genetic control of GLS biosynthesis in Indian mustard, including priority targets for further investigation and molecular marker development.
Keywords: Brassica juncea; genome-wide association studies; glucosinolates (GSL); seed quality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fine mapping of loci involved with glucosinolate biosynthesis in oilseed mustard (Brassica juncea) using genomic information from allied species.Theor Appl Genet. 2009 Feb;118(3):413-21. doi: 10.1007/s00122-008-0907-z. Epub 2008 Oct 21. Theor Appl Genet. 2009. PMID: 18979082
-
Dissection of genetic architecture for glucosinolate accumulations in leaves and seeds of Brassica napus by genome-wide association study.Plant Biotechnol J. 2020 Jun;18(6):1472-1484. doi: 10.1111/pbi.13314. Epub 2019 Dec 25. Plant Biotechnol J. 2020. PMID: 31820843 Free PMC article.
-
Seed glucosinolate yield is maximized by higher rates of sulfur nutrition than required for seed yield in condiment mustard (Brassica juncea L.).PLoS One. 2019 Apr 2;14(4):e0213429. doi: 10.1371/journal.pone.0213429. eCollection 2019. PLoS One. 2019. PMID: 30939141 Free PMC article.
-
Comparison of glucosinolate diversity in the crucifer tribe Cardamineae and the remaining order Brassicales highlights repetitive evolutionary loss and gain of biosynthetic steps.Phytochemistry. 2021 May;185:112668. doi: 10.1016/j.phytochem.2021.112668. Epub 2021 Mar 17. Phytochemistry. 2021. PMID: 33743499 Review.
-
Fire and Brimstone: Molecular Interactions between Sulfur and Glucosinolate Biosynthesis in Model and Crop Brassicaceae.Front Plant Sci. 2016 Nov 21;7:1735. doi: 10.3389/fpls.2016.01735. eCollection 2016. Front Plant Sci. 2016. PMID: 27917185 Free PMC article. Review.
Cited by
-
Identification of key genes controlling soluble sugar and glucosinolate biosynthesis in Chinese cabbage by integrating metabolome and genome-wide transcriptome analysis.Front Plant Sci. 2022 Nov 25;13:1043489. doi: 10.3389/fpls.2022.1043489. eCollection 2022. Front Plant Sci. 2022. PMID: 36507456 Free PMC article.
-
Genome-wide association study uncovers key genomic regions governing agro-morphological and quality traits in Indian mustard [Brassica juncea (L.) Czern. and Coss.].PLoS One. 2025 Apr 24;20(4):e0322120. doi: 10.1371/journal.pone.0322120. eCollection 2025. PLoS One. 2025. PMID: 40273405 Free PMC article.
-
QTL Mapping of Seed Quality Traits in Crops.Plants (Basel). 2025 Feb 6;14(3):482. doi: 10.3390/plants14030482. Plants (Basel). 2025. PMID: 39943043 Free PMC article.
-
Identification of Novel Loci Precisely Modulating Pre-Harvest Sprouting Resistance and Red Color Components of the Seed Coat in T. aestivum L.Plants (Basel). 2024 May 9;13(10):1309. doi: 10.3390/plants13101309. Plants (Basel). 2024. PMID: 38794380 Free PMC article.
-
Analysis of the quadruple lsu mutant reveals molecular determinants of the role of LSU proteins in sulfur assimilation in Arabidopsis.Plant J. 2024 Dec;120(6):2919-2936. doi: 10.1111/tpj.17155. Epub 2024 Nov 29. Plant J. 2024. PMID: 39612294 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

