Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum aestivum L.) Plants by Enhancing Root H+-Pump Activity
- PMID: 35161397
- PMCID: PMC8840481
- DOI: 10.3390/plants11030416
Synergistic Effects of Salicylic Acid and Melatonin on Modulating Ion Homeostasis in Salt-Stressed Wheat (Triticum aestivum L.) Plants by Enhancing Root H+-Pump Activity
Abstract
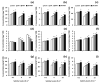
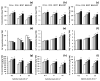
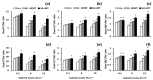
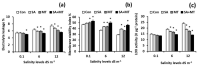
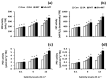
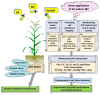
Salicylic acid (SA) and melatonin (MT) have been shown to play important roles in plant salt tolerance. However, the underlying mechanisms of SA-MT-interaction-mediated ionic homeostasis in salt-stressed plants are unknown. As a first investigation, this study aimed to clarify how SA-MT interaction affects H+-pump activity in maintaining the desired ion homeostasis under saline conditions and its relation to ROS metabolism. Wheat (Triticum aestivum L.) plants were grown under non-saline or saline conditions and were foliar sprayed with 75 mg L-1 SA or 70 μM MT. The SA+MT combined treatment significantly increased N, P, K+, Fe, Zn, and Cu acquisition, accompanied by significantly lower Na+ accumulation in salt-stressed plants compared to non-stressed ones. Additionally, it significantly enhanced ATP content and H+-pump activity of the roots. The mitigation was also detected in the reduced superoxide radical content, electrolyte leakage, and lipoxygenase activity, as well as increased superoxide dismutase, catalase, peroxidase, and polyphenol oxidase activities; K+/Na+, Ca2+/Na+, and Mg2+/Na+ ratios; relative water content; membrane stability index; and free amino acid accumulation in treated plants. The novel evidence shows that the higher root H+-pump activity in treated plants is a tolerance mechanism that increases the salt tolerance via maintaining ionic homeostasis.
Keywords: antioxidant response; melatonin; nutrient uptake; root H+-pump activity; salicylic acid; salt stress; wheat (Triticum aestivum L.).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Ashraf M.A., Asma H.F., Iqba M. Exogenous menadione sodium bisulfte mitigates specifc ion toxicity and oxidative damage in salinity-stressed okra (Abelmoschus esculentus Moench) Acta Physiol. Plant. 2019;41:187. doi: 10.1007/s11738-019-2978-7. - DOI
-
- Talaat N.B. Effective microorganisms improve growth performance and modulate the ROS-scavenging system in common bean (Phaseolus vulgaris L.) plants exposed to salinity stress. J. Plant Growth Regul. 2015;34:35–46. doi: 10.1007/s00344-014-9440-2. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

