A Survey of Enhanced Cold Tolerance and Low-Temperature-Induced Anthocyanin Accumulation in a Novel Zoysia japonica Biotype
- PMID: 35161412
- PMCID: PMC8839389
- DOI: 10.3390/plants11030429
A Survey of Enhanced Cold Tolerance and Low-Temperature-Induced Anthocyanin Accumulation in a Novel Zoysia japonica Biotype
Abstract
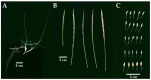
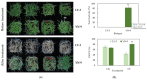
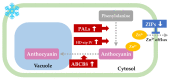
Zoysia japonica is a warm-season turfgrass that is extensively used in landscaping, sports fields, and golf courses worldwide. Uncovering the low-temperature response mechanism of Z. japonica can help to accelerate the development of new cold-tolerant cultivars, which could be used to prolong the ornamental and usage duration of turf. A novel Z. japonica biotype, YueNong-9 (YN-9), was collected from northeastern China for this study. Phenotypic measurements, cold-tolerance investigation, and whole-transcriptome surveys were performed on YN-9 and LanYin-3 (LY-3), the most popular Z. japonica cultivar in Southern China. The results indicated the following: YN-9 has longer second and third leaves than LY-3; when exposed to the natural low temperature during winter in Guangzhou, YN-9 accumulated 4.74 times more anthocyanin than LY-3; after cold acclimation and freezing treatment, 83.25 ± 9.55% of YN-9 survived while all LY-3 leaves died, and the dark green color index (DGCI) value of YN-9 was 1.78 times that of LY-3; in YN-9, there was a unique up-regulation of Phenylalanine ammonia-lyase (PAL), Homeobox-leucine Zipper IV (HD-ZIP), and ATP-Binding Cassette transporter B8 (ABCB8) expressions, as well as a unique down-regulation of zinc-regulated transporters and iron-regulated transporter-like proteins (ZIPs) expression, which may promote anthocyanin biosynthesis, transport, and accumulation. In conclusion, YN-9 exhibited enhanced cold tolerance and is thus an excellent candidate for breeding cold-tolerant Z. japonica variety, and its unique low-temperature-induced anthocyanin accumulation and gene responses provide ideas and candidate genes for the study of low-temperature tolerance mechanisms and genetic engineering breeding.
Keywords: RNA-seq; abiotic stress; anthocyanin biosynthesis; anthocyanin transport; turfgrass.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
The Effect of Ethephon on Ethylene and Chlorophyll in Zoysia japonica Leaves.Int J Mol Sci. 2024 Jan 29;25(3):1663. doi: 10.3390/ijms25031663. Int J Mol Sci. 2024. PMID: 38338942 Free PMC article.
-
Global Transcriptome Profiles of 'Meyer' Zoysiagrass in Response to Cold Stress.PLoS One. 2015 Jun 26;10(6):e0131153. doi: 10.1371/journal.pone.0131153. eCollection 2015. PLoS One. 2015. PMID: 26115186 Free PMC article.
-
Zoysia japonica MYC type transcription factor ZjICE1 regulates cold tolerance in transgenic Arabidopsis.Plant Sci. 2019 Dec;289:110254. doi: 10.1016/j.plantsci.2019.110254. Epub 2019 Sep 5. Plant Sci. 2019. PMID: 31623785
-
Chromosome-scale genome assembly of Zoysia japonica uncovers cold tolerance candidate genes.Sci Data. 2025 Apr 3;12(1):571. doi: 10.1038/s41597-025-04827-x. Sci Data. 2025. PMID: 40180989 Free PMC article.
-
Progress and Challenges in China Turfgrass Abiotic Stress Resistance Research.Front Plant Sci. 2022 Jun 14;13:922175. doi: 10.3389/fpls.2022.922175. eCollection 2022. Front Plant Sci. 2022. PMID: 35774814 Free PMC article. Review.
Cited by
-
Anthocyanin accumulation, inflorescence dry weight and total cannabidiol content have different temperature optima in Cannabis sativa.J Cannabis Res. 2025 Jul 29;7(1):51. doi: 10.1186/s42238-025-00311-w. J Cannabis Res. 2025. PMID: 40731014 Free PMC article.
-
Identification of new cold tolerant Zoysia grass species using high-resolution RGB and multi-spectral imaging.Sci Rep. 2023 Aug 14;13(1):13209. doi: 10.1038/s41598-023-40128-2. Sci Rep. 2023. PMID: 37580436 Free PMC article.
-
MdNAC104 positively regulates apple cold tolerance via CBF-dependent and CBF-independent pathways.Plant Biotechnol J. 2023 Oct;21(10):2057-2073. doi: 10.1111/pbi.14112. Epub 2023 Jun 30. Plant Biotechnol J. 2023. PMID: 37387580 Free PMC article.
-
Chromosome-level genome of Zoysia sinica in the intertidal zone reveals genomic insights into waterlogging stress adaptation.Plant Genome. 2025 Sep;18(3):e70070. doi: 10.1002/tpg2.70070. Plant Genome. 2025. PMID: 40629672 Free PMC article.
References
-
- Anderson S.J. Taxonomy of Zoysia (Poaceae) Morphological and Molecular Variation. Texas A&M University; Dallas, TX, USA: 2000.
-
- Shouliang C., Phillips S.M. Zoysia Willdenow, Ges. Naturf. Freunde Berlin Neue Schriften 3: 440. 1801, nom. cons. In: Zhengyi W., Raven P.H., editors. Flora of China. Science Press and Missouri Botanical Garden Pre; Beijing, China: St. Louis, MO, USA: 2013. pp. 496–498.
-
- Braun R.C., Milla-Lewis S.R., Carbajal E.M., Schwartz B.M., Patton A.J. Performance and playability of experimental low-input coarse-textured zoysiagrass in multiple climates. Grass Res. 2021;1:10. doi: 10.48130/GR-2021-0010. - DOI
-
- Meeks M., Kong S.T., Genovesi A.D., Smith B., Chandra A. Low-input golf course putting green performance of fine-textured inter- and intra-specific zoysiagrass (Zoysia spp.) Int. Turfgrass Soc. Res. J. 2021:1–12. doi: 10.1002/its2.63. - DOI
-
- Patton A.J., Schwartz B.M., Kenworthy K.E. Zoysiagrass (Zoysia spp.) history, utilization, and improvement in the United States: A review. Crop Sci. 2017;57:S-37–S-72. doi: 10.2135/cropsci2017.02.0074. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

