Wearable Electrochemical Sensors in Parkinson's Disease
- PMID: 35161694
- PMCID: PMC8839454
- DOI: 10.3390/s22030951
Wearable Electrochemical Sensors in Parkinson's Disease
Abstract
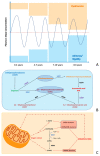
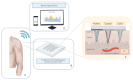
Parkinson's disease (PD) is a neurodegenerative disorder associated with widespread aggregation of α-synuclein and dopaminergic neuronal loss in the substantia nigra pars compacta. As a result, striatal dopaminergic denervation leads to functional changes in the cortico-basal-ganglia-thalamo-cortical loop, which in turn cause most of the parkinsonian signs and symptoms. Despite tremendous advances in the field in the last two decades, the overall management (i.e., diagnosis and follow-up) of patients with PD remains largely based on clinical procedures. Accordingly, a relevant advance in the field would require the development of innovative biomarkers for PD. Recently, the development of miniaturized electrochemical sensors has opened new opportunities in the clinical management of PD thanks to wearable devices able to detect specific biological molecules from various body fluids. We here first summarize the main wearable electrochemical technologies currently available and their possible use as medical devices. Then, we critically discuss the possible strengths and weaknesses of wearable electrochemical devices in the management of chronic diseases including PD. Finally, we speculate about possible future applications of wearable electrochemical sensors in PD, such as the attractive opportunity for personalized closed-loop therapeutic approaches.
Keywords: L-Dopa; Parkinson’s disease; biosensors; electrochemical monitoring; wearable sensors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

