Effects of Acute Yohimbine Hydrochloride Supplementation on Repeated Supramaximal Sprint Performance
- PMID: 35162339
- PMCID: PMC8835515
- DOI: 10.3390/ijerph19031316
Effects of Acute Yohimbine Hydrochloride Supplementation on Repeated Supramaximal Sprint Performance
Abstract
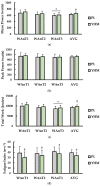
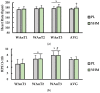
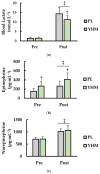
The purpose of this study was to examine the effects of a single acute dose of yohimbine hydrochloride on repeated anaerobic sprint ability. Physically active females (n = 18) completed two separate repeated supramaximal sprint trials each with a different single-dose treatment: placebo (PL; gluten-free corn starch) or yohimbine hydrochloride (YHM; 2.5 mg). For each trial, participants consumed their respective treatment 20 min before exercise. Following a warm-up, participants completed 3 × 15 s Wingate anaerobic tests (WAnTs) separated by 2 min of active recovery. A capillary blood sample was obtained pre- and immediately post-exercise to measure blood concentrations of lactate (LA), epinephrine (EPI), and norepinephrine (NE). Heart rate (HR) and rate of perceived exertion (RPE) were measured following each WAnT. Findings showed that mean power (p < 0.001; η2 = 0.024), total work (p < 0.001; η2 = 0.061), and HR (p < 0.001; η2 = 0.046), were significantly higher with YHM supplementation versus PL. Fatigue index (p < 0.001; η2 = 0.054) and post-exercise LA (p < 0.001; d = 1.26) were significantly lower with YHM compared to PL. YHM resulted in significantly higher EPI concentrations versus PL (p < 0.001; η2 = 0.225) pre- and post-exercise while NE only increased as a function of time (p < 0.001; η2 = 0.227) and was unaffected by treatment. While RPE increased after each WAnT, no differences between treatments were observed (p = 0.539; η2 < 0.001). Together, these results suggest that acute YHM ingestion imparts ergogenic benefits which may be mediated by lower blood LA and fatigue concomitantly occurring with blood EPI increases. Thus, YHM may improve sprint performance although more mechanistic study is warranted to accentuate underlying processes mediating performance enhancement.
Keywords: Wingate; epinephrine; lactate; norepinephrine; power output.
Conflict of interest statement
We have no conflicts of interest to disclose.
Figures
References
-
- Fu W.-Q., Li W., Wang J.-H., Du G.-H. Natural Small Molecule Drugs from Plants. Springer; Berlin/Heidelberg, Germany: 2018. Yohimbine; pp. 167–171.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

