Insights into the Potential Mechanisms of JAK2V617F Somatic Mutation Contributing Distinct Phenotypes in Myeloproliferative Neoplasms
- PMID: 35162937
- PMCID: PMC8835324
- DOI: 10.3390/ijms23031013
Insights into the Potential Mechanisms of JAK2V617F Somatic Mutation Contributing Distinct Phenotypes in Myeloproliferative Neoplasms
Abstract
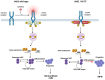
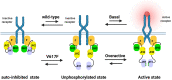
Myeloproliferative neoplasms (MPN) are a group of blood cancers in which the bone marrow (BM) produces an overabundance of erythrocyte, white blood cells, or platelets. Philadelphia chromosome-negative MPN has three subtypes, including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The over proliferation of blood cells is often associated with somatic mutations, such as JAK2, CALR, and MPL. JAK2V617F is present in 95% of PV and 50-60% of ET and PMF. Based on current molecular dynamics simulations of full JAK2 and the crystal structure of individual domains, it suggests that JAK2 maintains basal activity through self-inhibition, whereas other domains and linkers directly/indirectly enhance this self-inhibited state. Nevertheless, the JAK2V617F mutation is not the only determinant of MPN phenotype, as many normal individuals carry the JAK2V617F mutation without a disease phenotype. Here we review the major MPN phenotypes, JAK-STAT pathways, and mechanisms of development based on structural biology, while also describing the impact of other contributing factors such as gene mutation allele burden, JAK-STAT-related signaling pathways, epigenetic modifications, immune responses, and lifestyle on different MPN phenotypes. The cross-linking of these elements constitutes a complex network of interactions and generates differences in individual and cellular contexts that determine the phenotypic development of MPN.
Keywords: JAK2V617F; MPN.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Coexisting JAK2V617F and CALR Exon 9 Mutations in Myeloproliferative Neoplasms - Do They Designate a New Subtype?Asian Pac J Cancer Prev. 2016;17(3):923-6. doi: 10.7314/apjcp.2016.17.3.923. Asian Pac J Cancer Prev. 2016. PMID: 27039813 Review.
-
The effects of mutational profiles on phenotypic presentation of myeloproliferative neoplasm subtypes in Bosnia: 18 year follow-up.Bosn J Basic Med Sci. 2020 May 1;20(2):236-247. doi: 10.17305/bjbms.2019.4391. Bosn J Basic Med Sci. 2020. PMID: 31668145 Free PMC article.
-
CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable.Am J Clin Pathol. 2015 May;143(5):635-44. doi: 10.1309/AJCPUAAC16LIWZMM. Am J Clin Pathol. 2015. PMID: 25873496
-
CALR, JAK2 and MPL mutation status in Argentinean patients with BCR-ABL1- negative myeloproliferative neoplasms.Hematology. 2018 May;23(4):208-211. doi: 10.1080/10245332.2017.1385891. Epub 2017 Oct 9. Hematology. 2018. PMID: 28990497
-
Changing concepts of diagnostic criteria of myeloproliferative disorders and the molecular etiology and classification of myeloproliferative neoplasms: from Dameshek 1950 to Vainchenker 2005 and beyond.Acta Haematol. 2015;133(1):36-51. doi: 10.1159/000358580. Epub 2014 Aug 7. Acta Haematol. 2015. PMID: 25116092 Review.
Cited by
-
Metabolic Biomarkers Affecting Cell Proliferation and Prognosis in Polycythemia Vera.Cancers (Basel). 2022 Oct 7;14(19):4913. doi: 10.3390/cancers14194913. Cancers (Basel). 2022. PMID: 36230834 Free PMC article.
-
Blood metabolic and physiological profiles of Bama miniature pigs at different growth stages.Porcine Health Manag. 2022 Aug 8;8(1):35. doi: 10.1186/s40813-022-00278-7. Porcine Health Manag. 2022. PMID: 35941611 Free PMC article.
-
Discovery and biological evaluation of a novel and highly potent JAK2 inhibitor for the treatment of triple negative breast cancer.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2488127. doi: 10.1080/14756366.2025.2488127. Epub 2025 Apr 29. J Enzyme Inhib Med Chem. 2025. PMID: 40298145 Free PMC article.
-
Contribution of lowered hemoglobin threshold value in the diagnosis of polycythemia vera: Comparison of 2016 and 2008 WHO criteria.Medicine (Baltimore). 2023 Aug 4;102(31):e34462. doi: 10.1097/MD.0000000000034462. Medicine (Baltimore). 2023. PMID: 37543796 Free PMC article.
-
Exploring hematological alterations and genetics linked to SNV rs10974944 in myeloproliferative neoplasms among Amazon patients.Sci Rep. 2024 Apr 24;14(1):9389. doi: 10.1038/s41598-024-60090-x. Sci Rep. 2024. PMID: 38654055 Free PMC article.
References
-
- Levine R.L., Wadleigh M., Cools J., Ebert B.L., Wernig G., Huntly B.J.P., Boggon T.J., Wlodarska I., Clark J.J., Moore S., et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;4:387–397. doi: 10.1016/j.ccr.2005.03.023. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

